COVIDvax Correlates of Protection and Validation
September 29 | Posted by mrossol | Malone, Math/Statistics, Medicine, PharmaThe CDC, Press, and New England Journal of Medicine are clueless
Source: COVIDvax Correlates of Protection and Validation
Usual disclaimer: The opinions in this essay are my own, and not necessarily those of the US Government, Centers for Disease Control and Prevention (CDC), or the Advisory Committee on Immunization Practices (ACIP).
Warning: The following essay contains geeky material and insider regulatory terminology. If this is not your cup of tea, please pass it by. I usually write for general audiences, but in this case my target audience is more specialized and specific. For instance, my fellow ACIP members, CDC personnel, physicians, scientists, and Regulatory Affairs professionals. Hopefully, most FDA and Reg Affairs folks already know this stuff.
First off, please forgive the CDC communications staff for not recognizing the somewhat geeky regulatory term “Correlate of protection” and mistaking it for “correlative protection”.
I admit that I got triggered a couple of times at the September 2025 ACIP meeting, and one of those is when I could no longer tolerate sloppy scientific communications and conclusions from CDC staff during their presentations. In the case at hand, the issue involved assertions that production of antibody responses elicited by injecting an mRNA-based genetic delivery product, thereby causing cells of the body to manufacture spike protein and subsequently develop anti-spike antibodies, equates to protection from COVID. You can listen to a clip of this exchange below.
What is a “correlate of protection” and why should you care?
In the FDA’s guidance to industry titled “Product development under the animal rule”, a correlate of protection is defined as a measurable immune marker that is statistically related to a clinical endpoint and is reasonably likely to predict protection against disease. What this means in more practical terms is that if one can rigorously, statistically, and prospectively establish that some clinical marker and associated specific assay that you develop and test can measure something that predicts protection from a clinical outcome of an infectious disease, for example hospitalization after infection with SARS-CoV-2, then for purposes of licensure you can substitute that measurement for clinically measuring the desired clinical outcome (hospitalization risk after infection with SARS-CoV-2).
Why does this matter? Because statistically demonstrating that the drug produces the desired clinical effect (prevention of hospitalization) is expensive, time-consuming, risky and quite challenging in terms of study design, implementation, and analysis of an actual clinical trial to prove the “efficacy” of the product for that purpose (“indication”). Here is a practical example- if you want to develop a new influenza vaccine, there is a “validated correlate of protection” laboratory test surrogate for efficacy. It is called the hemagglutination inhibition (HI) assay. For influenza, a hemagglutination inhibition (HI) titer of 1 is commonly accepted as correlating with approximately 50% protection in adults. In the case of pneumococcal vaccines, an antibody concentration of 0.35 µg/ml measured by ELISA has historically been used as a correlate of protection, but this has not been rigorously, statistically demonstrated, and therefore is not a validated correlate of protection. Get this point? Just because, in general, some laboratory test outcome seems to be associated with a clinical outcome for a specific vaccine is not the same thing as verifying that is the case using a specific, well-controlled laboratory assay.
A validated correlate of protection may be specific to a particular vaccine type and not applicable to all vaccines protecting against a specific disease. This is because the “correlate of protection” does not necessarily measure the mechanism that is providing the protection from disease. It is only correlated to protection. And we all know that correlation is not proof of causation. This is why one is required to go through a rigorous, stepwise, and prospective statistical testing process to establish that a specific lab test actually correlates with protection. Otherwise, all you have is some version of an expert opinion or a best guess. And “expert opinions” have been proven to be the lowest quality of clinical evidence.
That is basically what the New England Journal of Medicine has published in 2022.
In the case of COVID, the definition of the disease almost completely overlaps with most other upper respiratory virus disease symptoms. Therefore, the clinical diagnosis of COVID is quite subjective. In other words, very subject to current trends, influenced by media reports, belief systems and biases of those doing or evaluating the diagnoses, etc. Symptoms observed during a period where there is widespread promoted fear of COVID are more likely to be diagnosed as COVID, but the same symptoms observed during a period where there has been widespread promotion of fear of Influenza are more likely to be diagnosed as Influenza. Which is why it is absolutely necessary to couple a clinical diagnosis of COVID to a laboratory test.
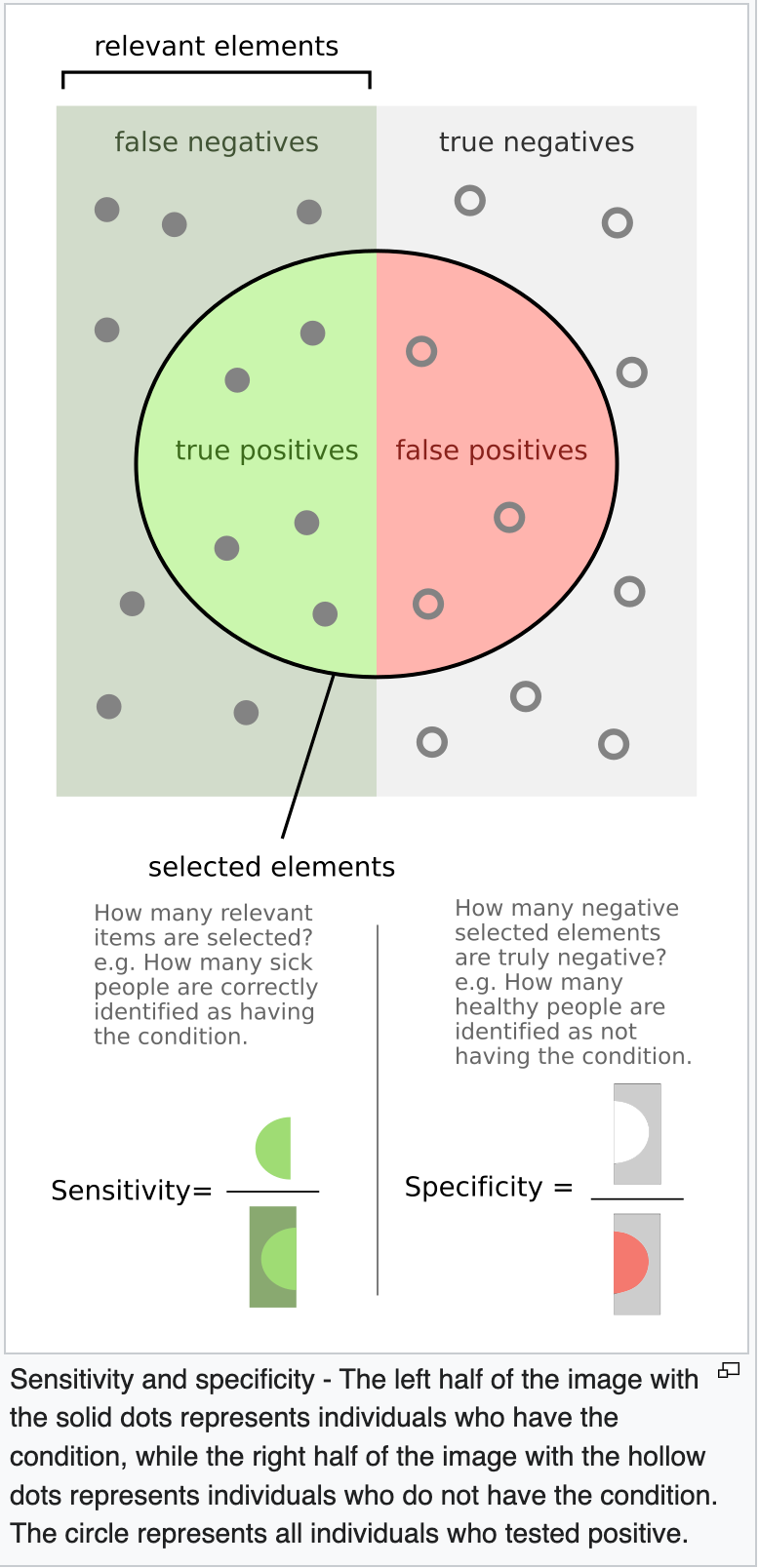
The predictive accuracy (positive predictive value, negative predictive value) of laboratory tests are a function of how prevalent the condition is in the population at the time of testing. From this, you can appreciate that any population-based modeling or analysis of disease frequency is also fairly subjective. The clinical diagnosis is subjective and influenced by external social factors such as media hype (note the still unexplained disappearance of clinical influenza during the COVID years). And the sensitivity and specificity of the laboratory detection of the footprint of the pathogen is also influenced by its actual prevalence (frequency) in the population.
- True positive: Sick people correctly identified as sick
- False positive: Healthy people incorrectly identified as sick
- True negative: Healthy people correctly identified as healthy
- False negative: Sick people incorrectly identified as healthy
The notorious “high cycle threshold” used in some COVID tests resulted in great sensitivity (presence of SARS CoV-2 viral RNA readily detected), but lousy specificity (lots of other RNA that did not come from SARS CoV-2 virus were falsely concluded to be from SARS CoV-2 virus.). Furthermore, SARS CoV-2 virus RNA fragments persist for weeks, so if you had a subclinical infection with SARS CoV-2 and recovered, but then developed a clinically severe influenza virus infection, you could still legitimately have a true positive PCR test for SARS-CoV-2.
And of course, not all cases of infection with SARS-CoV-2 caused severe disease. In most cases, as with most upper respiratory single-stranded RNA viruses, the disease caused is mild, self-limiting, and not life-threatening.
From this discussion, it is clear that laboratory analysis of viral diseases is a complex and challenging process. And by the way, those statistics on COVID deaths per day that we were all fed by the media during the COVIDcrisis? All based on modeling, not actual verified deaths from COVID. And even if they had been based on “verified” diagnosed deaths FROM COVID (rather that WITH a positive PCR test for SARS-CoV-2).
Aren’t antibodies what protects us from disease caused by viruses? And what is meant by “antibody titer” or “neutralizing antibody titer”?
This gets complicated.
The short answer to the first question is “sort of”. First off, at the simplest level, antibodies are a complex mixture of a type of protein that your immune system (B cells) produce that can recognize and attach to different parts of virtually any molecule that is large enough to be caught by the binding part of an antibody. You can think of antibodies as being like tableware – a spoon or a fork. They have a handle (Fc domain) and a business end that grabs things (the tines of the fork = Fab domain). But unlike tableware, they are highly variable, they get modified to have different activities at different points in the cycle of response to an infection, and each antibody of a different type will attach to a different small region of a virus.
Some antibodies bind to areas of a virus that will block the ability of the virus to infect a cell. These are called neutralizing antibodies. Some antibodies can bind to areas of a virus that can improve its ability to infect certain cells that bind antibodies. These are called enhancing antibodies. Most antibodies against a virus bind to parts of the virus that have no relationship to infection. We can call these last two groups non-neutralizing antibodies. Non-neutralizing antibodies can compete with neutralizing antibodies and reduce their ability to neutralize a virus.
Some believe that the main function of antibodies during a viral infection are not to block infection, but rather to clean up and eliminate cell-free virus and viral fragments that would otherwise drive excessive inflammation (in the extreme case, “cytokine storm”). Most viral infection is caused by cell-to-cell spread. And what immune response activity controls cell-to-cell spread? Primarly adaptive cellular responses (T cells, cytotoxic T cells etc.).
So, why do we measure total antibody levels produced by a vaccine, when most probably the diverse mix of antibodies have very little to do with controlling the infection and determining the course of disease? Because we have an easy test that detects total antibodies that bind to a virus or a virus fragment – it is cheap, fast, easy, and very non-specific. It is called the ELISA test (enzyme linked immunosorbent assay). But when someone (say the FDA, for example) tells you that some new vaccine booster is going to be effective in protecting you against hospitalization and death just because it causes some animal, or even a limited number of human subjects, to make a total antibody titer, you should immediately know that you are being misled. Or conned.
Total ELISA titer is almost completely useless as a predictor of efficacy or effectiveness of a vaccine against a respiratory RNA virus. Asserting, based on a small number of rodents injected with the product, that the fact that mice mounted a substantial ELISA response is predictive of disease protection in humans is an insult to the intelligence of patients, physicians, and public. And yet this was repeatedly done during COVID. This was a transparent con to anyone with any relevant experience of expertise. But Pharma and the FDA (CBER) promoted the con, corporate media repeated and socialized it, and the censorship-industrial complex did what it could to destroy the reputation and legitimacy of any who dared question the con.
So why not test T cell responses? Because those assays are hard to do at scale, and quite expensive. And the truth is that protection from disease relies on the full portfolio of adaptive (T and B cell) and innate immune system response. But to measure all of that is really, really complicated, expensive, and the truth is that we do not completely understand how the immune system interacts to provide for adaptive immunity.
What is a validated correlate of protection for a vaccine?
Under virtually all modern international regulatory authorities, establishment of a validated correlate of protection requires a series of rigorous steps and tests be performed.
The animal rule pathway for defining a correlate of protection, mentioned above, applies when an animal model exists or has been developed that adequately mimics the pathophysiology of a human condition, a product’s mechanism of action is the same in both animals and humans, and data obtained by use of that model support the selection of an effective human dose. None of these criteria exist for COVID or the COVID “vaccine” products. Therefore, the Animal Rule and the processes for using the animal rule do not apply in the case of COVID.
When I asked the CDC representative who was spouting nonsense about antibody titers and protection from COVID whether there was a validated correlate of protection from COVID, I already knew the answer. I was testing whether this CDC “expert” knew whether or not what she was saying had any merit. And she failed. So therefore all that she said in that presentation needs to be taken with a grain of salt. She was clearly not an expert. I might as well be listening to a pharmaceutical company sales rep mouthing the talking points the rep had been trained to recite. Actually worse- having been trained in Medical Affairs, I also know that no pharma rep would make those kinds of claims due to the associated legal liability.
The validation process typically includes several key steps and data sources.
First, natural history studies can identify correlations between immune markers and infectious disease outcomes in unvaccinated individuals, providing a baseline for what level of immunity might be protective. Second, individual-level analysis from a randomized, controlled vaccine efficacy trial is crucial, where the immune response in vaccinated individuals is compared to clinical outcomes to establish a statistical association.
Third, population-level analysis of a series of randomized, controlled trials or observational studies can strengthen the evidence by demonstrating consistency across different populations and settings.
→ This is basically where the NEJM publication mentioned above stops. Then the really hard work starts.
Post-approval vaccine effectiveness studies are also vital, as they confirm clinical outcome effectiveness and provide further data for CoP evaluation. For vaccines like the meningococcal C conjugate (MCC) vaccine, validation has been achieved by comparing efficacy estimates from postlicensure surveillance with the percentage of individuals achieving specific antibody titers, such as rSBA titers ≥4 or ≥8 at 4 weeks post-vaccination, which were found to be most consistent with observed efficacy.3 However, it is important to note that protection may rely more on immunologic memory than on a single antibody level at the time of exposure, especially for longer-term protection.
The use of standardized assays is critical for establishing a globally applicable CoP. For example, the lack of standardization in neutralizing antibody assays for SARS-CoV-2 has prevented the establishment of a universally accepted international threshold. In the absence of a standardized assay, regulators often rely on immunobridging studies, which compare the immunogenicity of a new vaccine to that of an existing, efficacious vaccine. These studies define noninferiority or superiority margins based on the geometric mean titer (GMT) of the established vaccine, using models derived from previous breakthrough infection studies to predict efficacy.
Statistical frameworks are also employed to formally estimate protective thresholds. The a model, for instance, uses profile likelihood or least squares methods to estimate a threshold that differentiates protected from susceptible individuals, with significance testing and confidence intervals obtained through modified likelihood ratio tests and bootstrapping. This formal approach helps move beyond visual inspection of data and provides a rigorous basis for defining thresholds.
Finally, the FDA has historically described four levels of CoPs, ranked by the strength of correlation with clinical endpoints, with level 1 representing the strongest, often involving a direct, mechanistic cause/effect relationship between the immune marker and protection.6 The ultimate goal is to establish a CoP that is not only statistically robust but also mechanistically plausible and applicable across different vaccine platforms and populations.
There are no statistically validated correlates of protection for COVID-19 disease. To assert otherwise is misleading and fraudulent. At this point, medical and clinical science cannot even define what makes COVID-19 different from any other upper respiratory virus disease. So the CDC, FDA, Pharma, and Dead Media should stop misleading the public about this.
Next I will discuss the biodistribution data fraud, a topic that I also brought up at the last ACIP meeting in a rather brisk discussion involving both Pfizer and Moderna representatives. The outcome was indeed interesting and most fascinating.


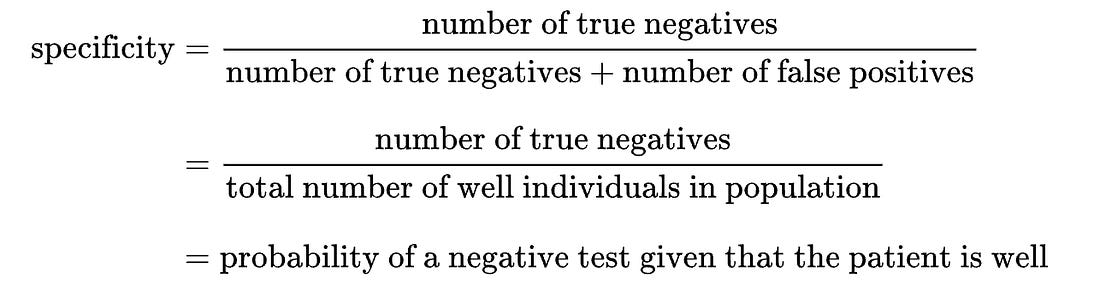






Leave a Reply
You must be logged in to post a comment.