AVIAN FLU PANDEMIC OR PANDEMONIUM? False Positives from Non‑Quantitative RT‑PCR on SARS‑CoV‑2. AIV H5 RT‑qPCR Is Set to Repeat the Same Catastrophe.
December 1 | Posted by mrossol | CDC NIH, Experts, Math/Statistics, ScienceThe Pandemaniacs Are Everywhere. Proper standards for nucleic acid testing will keep them at bay. The time to act is NOW. Tomorrow, it could be too late.
James Lyons‑Weiler, PhD
President, IPAK | Founder IPAK-EDU.org | Founder, NAATEC
The settings for a COVID 2.0 Pandemic of False Positives are all in place. “We must catch every case” is no excuse to misdiagnose individuals and let them cook and potentially die at home quarantined w/untreated, misdiagnosed bacterial pneumonia or other less virulent respiratory illnesses.
We could have saved millions and millions of lives if people had understood and acted in April 2020: False positives in PCR tests drove the COVID-19. We must not allow a repeat with avian flu.
In 2020, I warned—publicly, repeatedly, in articles, podcasts, and tweets, and with evidence, fighting censorship all the way—that using non‑quantitative RT‑PCR as the primary driver of pandemic policy would guarantee a tidal wave of false positives, distort epidemiology, and weaponize diagnostic noise as public fear. Those warnings were not vague or speculative; they were precise, technically grounded, peer‑reviewed, and absolutely correct.
I explained that without internal negative controls for Ct‑stratification, nested PCR confirmation, or sequencing, PCR tests would be repurposed into fear‑amplifiers rather than disease‑detectors. I warned that once governments built policy on raw PCR counts and arbitrary Ct values, no one would be able to distinguish real outbreaks from diagnostic artifacts. I said we would lose the ability to tell signal from noise, disease from contamination, and epidemiology from hysteria. I knew I was right. But too few could understand how central the diagnostic grift was the COVID-19 fear mongering.
People in high places heard the warnings. They understood them. I know, because I warned Peter Marks at US F.D.A. And others.
And he and the others who knew did nothing. Millions died after developing severe, untreated, misdiagnosed bacterial pneumonia.
That inaction helped create a world where some actors benefited from chaos—whether through political leverage, pharmaceutical opportunism, or supranational control frameworks. Call them what they are: enemies of stability who thrive when populations panic.
I warned too early. Nothing happened.
But then they came after all of our jobs. All of them. That got our attention. But cataclysmic damage was already done, including millions of deaths due to misdiagnosed and untreated bacterial pneumonia and sepsis.
We Must Call them “PANDEMANIACS”
Now, those same forces stand ready to exploit the next diagnostic mirage. Pandemaniacs are all over Twitter, Bluesky, everywhere posting one-off references to H5N1 as an inevitable next pandemic.
Standard H5/AIV RT-qPCR assays include NTCs, negative extraction controls, and internal positive controls, though they do not include a true sample-matched internal negative template.
Instead, they rely on fixed Ct thresholds (usually ~35–38 depending on the lab/kit) and internal positive controls to assess severity of, not yes/no, infection.
Ct cutoffs are supposed to originate from analytical LoD validation and per-sample control and to thereby compensate for variable starting material; despite this, labs still use them as binary yes/no decision points rather than quantitative measures in spite of the fact they do not adjust for variation in starting material on swabs. The concern, of course, is non-specific amplification.
They have a No-template control (NTC) run separately to detect contamination, but that is not useful. A matching negative control source is needed for off-site amplification assessment. Or, sequencing. This is a NON-NEGOTIABLE.
Unless we act immediately and forcefully, AIV H5 RT‑qPCR will repeat—and possibly exceed—the PCR‑driven chaos of COVID‑19.
We must hold the line: NO PROOF OF SEQUENCE? NO DIAGNOSIS. NO DIAGNOSIS? NO PANDEMIC.
What I Showed Then: False Positives Were Always the Core Threat
My 2021 paper The Balance of Risk in COVID‑19 Reveals the Extreme Cost of False Positives demonstrated mathematically that even a 1% false‑positive rate in low‑prevalence settings would lead to double‑digit misclassification. That is not a hypothesis. That is arithmetic any molecular biologist familiar with the arbitrariness of RT-PCR to the amount of starting material and any epidemiologist should have respected.
Then came the empirical proof: Dr. Sin Hang Lee—one of the most masterful and rigorous molecular diagnosticians alive—verified PCR positives using nested RT‑PCR followed by Sanger sequencing. In multiple studies, he found:
- Over 40% of RT‑qPCR “positives” failed sequence confirmation in real‑world panels.
- Some panels showed complete absence of SARS‑CoV‑2 RNA despite PCR positivity.
- Contamination and mis‑priming were rampant at high Ct values.
Those results were not anomalies—they were the structural consequence of relying on non‑quantitative PCR for mass screening.
I echoed those warnings in Follow the Science, Not Mere Authority on PCR False Positives, and NAATEC formalized the solution: nested RT‑PCR+Sanger sequencing as the gold standard.
But officials and institutions stayed silent. They knew the risks. They understood the mechanics. They failed to act. Intentionally.
And that failure built the diagnostic culture we now inhabit—a world where raw PCR counts are treated as unquestionable truth.
Japan: The Internet Epidemic With No Sequencing Backbone
Right now, Japan’s influenza surge is being blasted across the global internet in real‑time updates—case counts, hospitalization numbers, fear‑driven commentary, and nonstop amplification by outbreak‑tracker accounts. None of these posts include Ct values, assay parameters, or sequencing confirmation.
This is the same diagnostic opacity that drove global chaos during COVID‑19, now reappearing in the influenza domain—precisely when governments, media, and supranational institutions are primed to react.
Meanwhile, a single gull in a bioRxiv paper was sequenced to clade 2.3.4.4b with proper molecular rigor. A bird. A tick. Full lineage assignment.
If a single bird receives more diagnostic rigor than thousands of human “cases,” you are not watching epidemiology—you are watching policy by unverified fluorescence.
And if informed people remain silent this time, the enemies who weaponize fear will win again.
No Sequence, No Case Count. No Nested Confirmation, No Pandemic Curve.
This is the line.
This is the standard.
This is the bright red boundary that must not be crossed again.
If sequencing is not performed, then PCR positives are NOT clinical cases, NOT epidemiological evidence, and NOT a valid basis for public‑health actions.
Therefore, we must insist on:
- 100% nested RT‑PCR + Sanger sequencing of all early outbreak samples until ≥300 true positives are confirmed.
- 2 to 20% ongoing sequencing confirmation, stratified across Ct bands (<25, 25–30, 30–35, >35), laboratories, and sample types to provide N>1000 empirical votes on SN, SP, FPR, and FDR.
- Full disclosure of Ct distributions, LoD, assay design, primer/probe sequences, and sequencing confirmation rates.
- Immediate audits of any laboratory with a confirmation rate <80% in any sample category.
- Mandatory sequence deposition in open databases.
If a lab cannot meet these standards, it should not be generating case counts. Period.
Sanger Sequencing: A Simple, Scalable Audit for PCR-Derived Case Counts
The critical corrective to RT‑qPCR’s false‑positive risk is embarrassingly simple and already available in virtually every diagnostic lab: nested PCR plus Sanger sequencing. This combination converts each “positive” from a mere fluorescence signal into a bona fide genomic identity.
Why this works — and is easy
- Use the same RNA extract submitted for routine RT‑qPCR.
- Run a nested PCR using primers targeting a longer, highly conserved region (≥ 350–450 bp). Not every test. Just thousands to know the FPR and the FDR.
- Purify the amplicon and perform Sanger sequencing (cost ≈ USD 6–12 per sample).
- Align sequence output to reference genome.
- A clean match = verified infection.
- No match or ambiguous sequence = false positive, likely assay noise or contamination.
- No new platforms. No exotic reagents. No additional infrastructure beyond standard molecular‑biology resources.
All hospitals and molecular labs worldwide already have what it takes. This is not futuristic — this is routine molecular diagnostics.
The 14 % Reality Check: What Happens When You Do the Audit
A recent re‑analysis of a nationwide dataset (the German “ALM” consortium, which handled ~90% of the country’s SARS‑CoV‑2 PCR testing) found that when cumulative RT‑PCR positives were compared against later IgG seroconversion data, the scaling factor that best fit the observed antibody curves was 0.14 — meaning only ~14% of PCR-positive individuals ever developed detectable antibodies, consistent with actual infection. (NB: The 14% µ parameter reflects aggregate PCR-to-IgG calibration and includes repeated testing, IgG sensitivity, and sampling bias—not solely false positives.) Frontiers
In other words — when one applies a biological endpoint (seroconversion) rather than a fluorescence threshold — about 86% of PCR positives failed to represent true infections.
This dramatic finding collapses the inflated case curves we were shown in 2020–2021 into a far smaller, biologically plausible pandemic.
It aligns with several well-documented mechanisms of error: non‑specific amplification, environmental contamination, primer mismatches, RNA fragments, and background noise — all of which are exactly the pitfalls sequence confirmation circumvents. Cureus
Sensitivity Decay Over Time — Another Reason to Sequence, Not Trust Ct
Beyond false positives, RT‑qPCR’s sensitivity (true positive detection) degrades over time with SARS‑CoV‑2 evolution and biological dynamics. A study of 644 suspected COVID-19 patients found that while early after symptom onset sensitivity ranged 80–95%, it fell rapidly in mild cases as infection progressed. PMC
Meanwhile, viral evolution has repeatedly altered primer/probe binding sites, undermining assay performance unless continuously re‑validated and re‑designed. PMC
Thus: as the virus evolves and our sensitivity erodes, false negatives rise — but without sequencing or repeat testing you’ll never know. In tandem with the high false‑positive risk, this combination makes raw PCR counts almost meaningless.
Due to molecular evolution, the primer set involving the S-gene in the SARS-CoV-2 virus dropped out. This caused the local COVID PCR kits to drop of sensitivity in the UK to 50% for 8 months until the problem was found and the rule was changed to ignore the S-gene involved primer pair. Andrew Rambaut, in a most ad-hoc manner, celebrated that, after 8 months of 1/2 of the positive people walking away thinking they were negative spreading “The UK variant” (unbeknownst to health officials) the loss of S-gene primer reporting could be used to distinguish variants. Poppycock.
It was late 2020, as SARS-CoV-2 mutated away from the original RT-PCR primers, laboratories across the United Kingdom discovered what they called “S-gene target failure” (SGTF)—a failure of PCR assays to amplify the spike gene target, while other gene targets remained positive. This phenomenon wasn’t immediately seen as cause for alarm— and was detected 8 months after it started.
Instead of recognizing this as a collapse in sensitivity, officials treated it as a data anomaly. Public Health England and academic researchers, including Andrew Rambaut, retroactively celebrated the S-gene dropout as a useful feature—it helped distinguish a new lineage, soon dubbed the “UK variant” or B.1.1.7 (later Alpha).
But what this reframing ignored was the public health consequence of an 8-month gap: an unrecognized window during which a large number of infected individuals were incorrectly told they were negative due to broken primer binding—despite being contagious. Mathematics showed that RT-PCR sensitivity for that S-gene-targeted assay fell to ~50% against the emerged variant, effectively doubling the false-negative rate in critical settings like hospitals, care homes, and community testing programs.
Instead of issuing a nationwide alert and updating assay design, UK officials leaned into the narrative: we can detect the variant precisely because the S-gene fails to amplify. In other words, a defect was floated as a diagnostic feature.
This ad-hoc rationalization reveals the danger of allowing policy to adapt to assay failures rather than correcting them. The proper response would have been:
- Immediate identification and sequencing of all S-gene dropout samples.
- Urgent revalidation of all RT-PCR assays using the latest circulating sequences.
- Transparency about the loss of sensitivity and the risk of false negatives.
Instead, silence prevailed, and a preventable spread event was reframed as an accidental innovation.
This is exactly the kind of narrative inversion that a live sequencing audit—like the one demanded throughout this article—would have exposed and corrected in real time. We cannot allow another pathogen, another primer set, or another population to suffer under the same negligent improvisation.
We already have the tools to distinguish real infection from PCR mirage. Nested PCR + Sanger sequencing is cheap, rapid, and universally available. And when used, it exposes the truth:
A recent major analysis showed that while 89% of early COVID‑19 PCR positives represented real infections (which is a disaster for screening) only 14% of later PCR positives could be validated biologically. The virus evolved away from the assay, and because authorities refused to implement sequencing audits, sensitivity decayed silently.
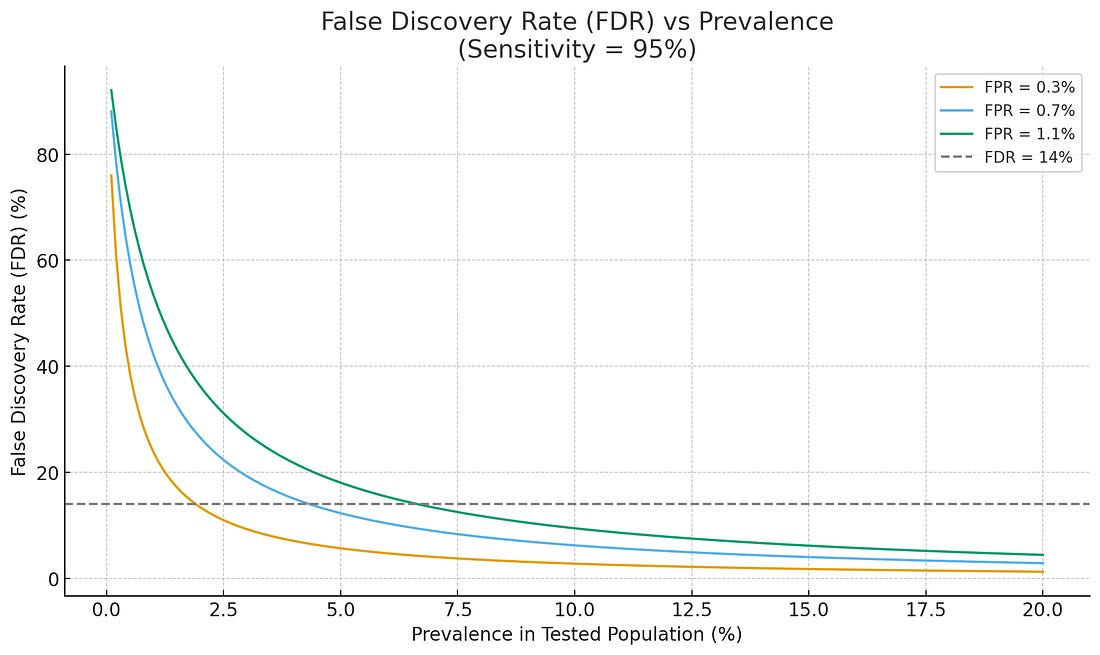
Figure. At low-prevalence, zero false positives are needed to prevent pandemonium.
We could have measured this drift in real time. We chose not to. That silence fueled chaos. And killed untold millions.
Ongoing Audit: How to Monitor False Positives (and Sensitivity Drift) in Real Time
Technical studies that show low false positives are misleading as they do not reflect real-world data.
EQA “0.1–1% false positives” is not an empirical measure of real-world diagnostic accuracy. It is an artifact of idealized testing conditions that omit the dominant drivers of PCR false positives: late-Ct noise, contamination pressure, sample-to-sample variation in starting material, sample-collection error, primer drift, and low-prevalence FDR explosion. EQA data cannot be used to claim PCR specificity in population-scale screening.
EQAs measure specificity, not False Discovery Rate (FDR) in Low Prevalence
FP Rate (FPR) is not the operationally relevant statistic for screens, and everyone in molecular diagnostics know this
During low-prevalence mass screening:
Specificity of 99% → FDR > 50%
Specificity of 99.9% → FDR ~10%
This is arithmetic, not interpretation.
EQAs report technical specificity, not population-level FDR, which is the real driver of policy distortion.
Result: Even “0.1% FP” in controlled conditions can produce a majority of real-world positives being false when prevalence is low.
Note that:
- EQAs and clinical studies place true analytical false‑positive rates for SARS‑CoV‑2 RT‑PCR in the ≈0.1–1% range in properly run labs. Nature
- Real‑world false discovery rates in mass screening at low prevalence can be high even with that specificity; Cohen’s preprint shows easily >50% FDR in very low prevalence settings if you keep testing everybody. (Cohen and Kessel, 2020 preprint).
“We must catch every case” is no excuse to misdiagnose individuals and let them cook and potentially die at home quarantined w/untreated, misdiagnosed bacterial pneumonia or other less virulent respiratory illnesses.
Let’s be clear: any government, lab, or institution unwilling to implement this audit protocol is unfit to conduct population-wide surveillance. This is the baseline for credibility.
Rather than accept PCR case, severe case, hospitalization and death counts as fact, public‑health labs and agencies must implement continuous audit protocols:
- Require negative controls. Each kit. Every time.
- For each batch of RT‑qPCR positives, randomly select a defined proportion (e.g., 10–20%) for nested RT‑PCR + Sanger sequencing.
- Define a defensible differential diagnosis protocol to properly diagnose all patients.
- Stratify sampling by Ct value bands (e.g., < 25, 25–30, 30–35, > 35), by laboratory, and by sample type (clinical, environmental, screening) and published corrected case counts.
- See that the high FPR and FDR means most people testing positive are NOT positive and require OTHER medical care. Prevent deaths especially due to bacterial pneumonia.
- Publish weekly “PCR audit reports” summarizing:
- Fraction of PCR positives confirmed by sequencing,
- False-positive rate and false discover rates with confidence intervals,
- Observed sensitivity drift over time (e.g., proportion of known infections missed, or dropout of previously validated primer binding),
- Primer/probe sequence versions, Ct thresholds, dynamic range and limit-of-detection (LoD) data.
- Adjust all case, severe case, hospitalizations and deaths by the FDR.
- Trigger investigations or assay re‑validation if confirmation rate drops below a predetermined threshold (e.g., < 70% positive confirmation or > 2–3% false-positive rate) as the virus evolves away from the test.
- Require transparency in all details of each kit.
This approach transforms RT‑qPCR from a black‑box alarm into a measurable, audited surveillance tool.
Why This Matters for AIV H5 (and Future Threats)
If we lacked such an audit for SARS‑CoV‑2, we simply erred under crisis. But now — with mounting examples of overcount, evolutionary drift, false positives, and confirmed serology‑PCR mismatch — we cannot claim ignorance.
For H5N1 and other avian flu RT‑qPCR assays (or any nucleic‑acid test used at population scale), the same audit must be mandated before trusting results that trigger culling, trade bans, or mass panic.
- If a single gull and its ticks can be fully sequenced and lineage‑typed, then nationwide case counts deserve at least that level of rigor.
- Without nested PCR + sequencing audit, Ct‑based positives remain probabilistic noise — not data.
- Real public health requires measurement with accountability, not blind faith in fluorescence thresholds.
Without ongoing sequencing audits, PCR‑based surveillance becomes a guessing game—one ripe for exploitation by actors who thrive on uncertainty. A 20% sequencing‑audit rule provides real‑time false‑positive estimation, sensitivity tracking, and immediate detection of assay drift.
This is not optional. This is the minimum standard of scientific integrity.
PROPOSED HHS PCR Diagnostic Audit Protocol Template
Mandatory for Public Health Laboratories, Surveillance Networks, and Regulatory Bodies
Purpose: To empirically quantify and monitor false-positive rates and sensitivity drift in RT-PCR-based diagnostic and surveillance assays used for infectious disease detection (e.g., SARS-CoV-2, H5N1, MPXV, RSV, etc.).
1. Sample Confirmation Requirements
- URGENT: Require negative control runs for all kits labeled “qRT-PCR”. Otherwise, it should be labeled “Non-Quantitative RT-PCR Subject to Sequence Verification”
- For any RT-PCR-based assay contributing to public health surveillance or case definitions:
- During initial deployment, 100% of RT-PCR-positive samples must undergo nested RT-PCR followed by Sanger sequencing to confirm specificity. (See Basil et al. PMC for first empirical demonstration of FPs in COVID RT-PCR kits: 11%)
- In routine operation, a minimum of 20% of all RT-PCR-positive results must be randomly sampled and sequence-confirmed.
- All patients require a defensible differential diagnosis.
- Samples should be stratified across:
- Ct value ranges: <25, 25–30, 30–35, >35
- Laboratory origin
- Sample type (e.g., NP swab, blood, environmental)
- Clinical context (screening vs diagnostic)
- Case reporting etc. all must be adjusted downward from empirical estimates of the FDR.
2. Technical Specifications
- Nested RT-PCR amplicon length: ≥350 bp
- Sequencing platform: Sanger (preferred for per-sample clarity), or NGS for batch confirmation
- Sequence alignment: Primers must cover unique, conserved regions of the target pathogen with ≥98% identity to reference genome
- Positive confirmation: Defined by contiguous sequence alignment with expected gene region(s)
3. Reporting Requirements
- Weekly False-positive rate (FPR) and FDR, with 95% CI, across Ct strata
- Sensitivity decay or dropout trends (e.g., missed positives later confirmed by sequencing or serology)
- Primer/probe versions in use, LoD, and dynamic range
- Proportion of “positives” failing sequence confirmation
- Sequence uploads to open databases (GenBank, GISAID) with anonymized metadata
4. Corrective Action Triggers
- Investigate assays, reagents, or workflows if:
- FPR >2% for any Ct strata or lab
- Sequencing confirmation rate <80% in any sample category
- Sensitivity declines sharply compared to historical benchmarks
This protocol aligns with MIQE 2.0, ISO 20395, and post-EUA assay harmonization efforts. Its adoption ensures that diagnostic output remains a quantitative measurement, not a probabilistic inference, and that the public can trust that molecular test results are empirically confirmed — not politically assumed or enforced by narrative.
Every public‑health lab, academic center, veterinary diagnostic facility, and surveillance program must adopt a sequencing‑confirmed audit protocol. This prevents PCR from being misused as a panic‑generator.
Because panic serves someone. And that someone is not you.
Act Now — Because Inaction Helps Those Who Want Pandemonia (The Pandemaniacs)
Understand this clearly: If you are informed and you remain silent, your silence is functionally equivalent to helping the forces that want continuous crisis, unrestricted authority, and mass compliance.
Those who seek pandemonia rely on three things:
1. Diagnostic opacity
2. Unquestioned PCR results
3. A compliant public that never asks for proof
Break any one of these, and their power collapses.
So act now: – Demand sequencing confirmation from your health departments.
– Demand Ct transparency.
– Demand nested PCR workflows with sequencing
– Demand that AIV H5 not be allowed to become the next PCR‑driven mirage.-Ask your favor podcast host to have me on immediately.
Science is supposed to resolve uncertainty—not manufacture it.
We failed once. We do not get to fail again.
#EndTheMadness #NAATEC #PCR #FalsePositives #H5N1 #NoSequenceNoCase #PANDEMANIACS





Leave a Reply
You must be logged in to post a comment.