Kennedy Unchained
December 8 | Posted by mrossol | CDC NIH, FDA, MalonePOTUS has released his Secretary of Health to disrupt the vaccine-industrial-academic complex
Source: Kennedy Unchained – by Dr. Robert W. Malone – Malone News
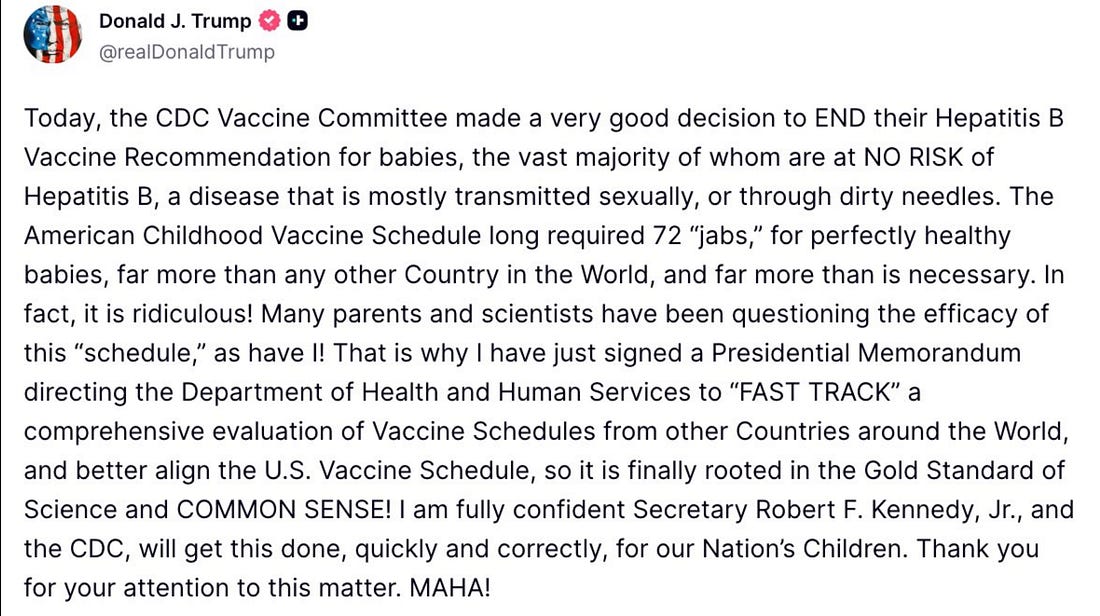
The President of the United States, Truth Social, Friday, 05 December, 2025
“Never wrestle with Pigs. Both you and the Pigs end up covered in mud, but the Pigs like it.”
Collected sayings of MIT Professor Retseif Levi, 2025
For some reason, the Academic/Biopharmaceutical Industrial Vaccine Complex (including the medical guilds) still believes that repeating propaganda, smearing, bullying, and gaslighting by those who disagree with their agendas and interpretations, as well as the American public, continues to be effective after COVID.
My sense is that there are cracks in the dyke, and they are running out of little Dutch boys to plug the leaks. Have patience, Brothers in Arms. The Roman Empire was not destroyed in a day. These things take time.
What I found particularly fascinating about the recent ACIP meeting was not the angry, strident few dominating discussions by the liaison organizations, but rather those that were silent. The silent ones included the representatives from the BIO industry association and from the National Foundation for Infectious Disease. I think that was a tell.
The Times They Are A-Changin’.
Without a doubt, the last few days have seen the most profound threats to the power of the academic-industrial-government vaccine complex in my lifetime. Billions of dollars of annually recurring, federally subsidized, monopolistic profit are suddenly going right down the drain. And I guarantee, they don’t like it very much.
It began with the lingering effects of the disclosure of documented, FDA-verified pediatric deaths reported to VAERS (and covered up) after administration of COVID mRNA vaccines. A complete vindication of Ernesto Ramirez Sr., who had toured the country to alert Americans about how he lost his son to myocardial damage from the Pfizer product.
Then, apparently triggered by this dark truthbomb, in a rough approximation of the immortal refrain yelled by anchorman Howard Beale in the 1976 film Network, the Director of the FDA Center for Biologics Evaluation and Research (Dr. Vinay Prasad) sent a memorandum out to all CBER staff basically saying he was mad as hell at the entire vaccine industry and it’s comprehensive epistemic capture, and he was not going to take it anymore.
As if that was not enough bad news, then the reconstituted, conflict-of-interest scrubbed Centers for Disease Control and Prevention Advisory Committee on Immunization Practices (CDC ACIP) met at the end of the week, and in a pair of nuanced advisory recommendations, voted to end the recommendations (functionally mandates) for Hepatitis B birth dose vaccination of all US-born children at low risk for contracting the virus. Few, if any, of our “colleagues” in the press appear to be able to read English, think, and appreciate the nuances of the approved language. Fewer still seem capable of processing the impact of the second approved recommendation.
The resulting howls of anger and outrage from both industry shills and US-based medical guilds were heard around the world. While pretty much the entire rest of the industrialized West, having long since rejected universal birth dose HepB Virus vaccination, was left scratching their heads, wondering what all the fuss was about.
So, let’s take just a moment to break those ACIP advisory votes down. Here is the final approved language. This is a copy of the actual final language document as circulated to the ACIP members prior to the votes last Friday.
General Principles
The CDC ACIP does not set federal vaccine policy. The CDC Director (or acting Director), whom the President appoints, sets policy. The ACIP advises the Director, who may disregard, modify, or accept the advice. So, despite the votes in favor of this language, nothing is official policy until the CDC Director says so.
The Preamble
Note that the woke language of “birthing persons” is nowhere to be found. Nor did it come up during any of the broadcast discussions. A minor win.
Breaking this down, beginning with the opening sentence. The ACIP Working Group responsible for reviewing the data and evidence was not asked to address potential changes to the current birth dose (and anti-HBs immunoglobulin) administration policies for those mothers who are infected with Hepatitis B, nor for those (very rare) cases where the Hepatitis B infection status of the mother is unknown. Only for mothers who are documented to be Hepatitis B negative. Therefore, the current recommendations stand, and it is still recommended that babies born to HBV-positive mothers be vaccinated at birth and receive immunoglobulin, as well as vaccination. Because these babies are clearly at significant risk for developing Hepatitis B infection.
Those press reports that the ACIP voted to entirely stop the HepB vax birth dose are disinformation.
Vote 1
The language approved in Vote 1 is quite nuanced. This only addresses infants born to mothers documented not to have Hepatitis B virus infection. As promised by Secretary Kennedy, no one has had their ability to obtain a government-subsidized HBV vaccine taken away.
line by line-
- “For Infants born to HbS negative women:” The risk of a newborn contracting Hepatitis B at or soon after birth is functionally zero if mom is not infected with the virus. The only way this can happen is exceedingly rare – that being that the newborn is exposed in a significant way to someone who is infected with Hepatitis B – typically blood or body fluid exposure. Yes, Hepatitis B can remain viable on various surfaces for a while. But the risk of a newborn acquiring “horizontal transmission” of Hep B in the USA is exceedingly rare.
- ACIP recommends individual-based decision-making, in consultation with a health care provider, for parents deciding when or if to give the HBV vaccine, including the birth dose. Parents should talk to their health care provider about what is best for their child, but the decision on WHEN OR IF to accept or reject the HBV vaccine is up to the parents. This means that any HepB Vax mandate, anywhere in the USA, is not consistent with the ACIP recommendation.
The ACIP not only voted to end the recommendation to vaccinate at birth in the case of low-risk pregnancies, but it also voted to enable parents/guardians to decide when or even if their children should receive this product. Another key point that the self-anointed arbiters of public health policy in corporate media seem to have overlooked.
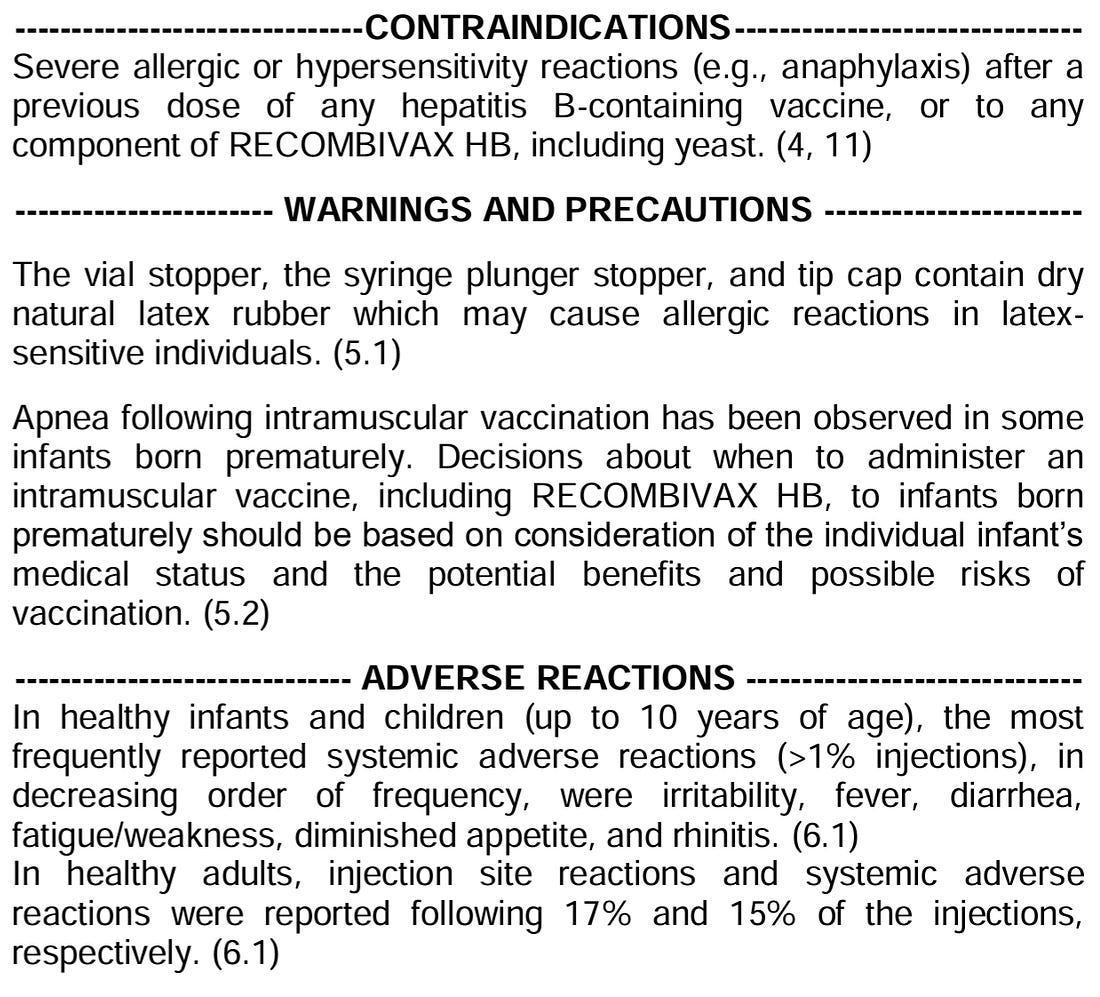
“Parents and health care providers should consider vaccine benefits, vaccine risks, and infection risks.” This sentence defines what health care providers need to document that they have discussed with parents. All three. That means for the children born of a Hepatitis B uninfected mother, parents need to be told that the risk of HepB to the newborn is close to zero. But it also means that, for parents living in communities where HepB is common, there needs to be a discussion that the risk of “horizontal transmission” is higher, and they should consider vaccinating their child for HepB. It also means that health care providers will have to explicitly discuss the risks and adverse events listed in the vaccine’s package insert. For example:
4. “For those not receiving the HBV birth dose, it is suggested (not RECOMMENDED!) that the initial dose is administered no earlier than two months of age.” So, high-risk infants or those born to mothers for whom HBV status is unknown (which “unknown” scenario at this point is essentially medical malpractice), are still recommended to receive a dose at birth. For all others, IF there is a decision by parents to vaccinate with one of the HepB vax products, the first dose should be deferred until the baby is at least two months old.
Vote 2
This one seems to be completely overlooked in press coverage, but it also passed. Yet it is far more revolutionary and disruptive than Vote 1, which pretty much just aligns US policies with those of leading Northern European public health positions.
Vote 2 has wide-ranging implications because it establishes a precedent that “booster dose” decisions should be individualized, based on actual data about a child’s prior immune responses. Looking forward, this also opens the door (or Overton window) to evidence-based management of those with “natural immunity” from prior exposure to an infectious agent, although not specifically for HBV.
This nuance seems to have slipped by the self-appointed vaccine and immunology experts reporting for MSM.
- “When evaluating the need for a subsequent HBV vaccine dose in children, parents should consult with health care providers to determine if post-vaccination anti-HbS serology testing should be offered.” This means that after dose #1 (the priming dose), the decision to proceed with subsequent boosting should be informed by what the baby/child’s immune response was to the prior dose.
- “Serology results should determine whether the established protective HbS titer threshold of ≥10 mIU/mL has been achieved.” A significant fraction of children tend to mount a robust immune response after the primary dose. Others require two doses, and still others require three doses. According to the Centers for Disease Control and Prevention (CDC), a post-vaccination anti-HBs titer ≥10 mIU/mL is associated with hepatitis B immunity. ABOUT 5% of CHILDREN DO NOT MOUNT A STRONG ANTIBODY IMMUNE RESPONSE EVEN AFTER THREE VACCINE DOSES. By current medical dogma, these kids would need a fourth dose?
- “The cost of this testing should be covered by insurance.” That clause is in there to ensure that Medicare/Medicaid (and under Obamacare, commercial insurers) will cover the cost of the anti-HBs titer testing. By the way, this will shift revenue from HBV vaccine manufacturers to hospitals and contract laboratories that perform anti-HBs titer testing. It will empower parents to make informed choices when deciding whether or not to “boost” their children, and will provide them with documentation of protective titer in case school districts, future universities or employers require documentation of vaccine-induced protection of the child. Which is far more scientifically sound than the current policies.
What Were the Chains on HHS Secretary Kennedy?
Senator Cassidy extracted promises from Sec. Kennedy regarding childhood vaccines. What were those promises?
- Kennedy promised he would not change the existing U.S. childhood vaccination schedule. Los Angeles Times
- He committed to maintaining the existing vaccine safety and approval systems, rather than creating new or parallel systems. Politico
- He said he would not undermine or restrict congressionally mandated funding for vaccination programs. AP News
- He agreed to provide advance notice to Congress if he planned to make any major changes to vaccine safety monitoring. Wikipedia
- He pledged to allow Cassidy direct involvement — namely, that Cassidy could nominate at least one candidate to the key advisory body that sets vaccine recommendations (the ACIP). Reuters
- Kennedy further promised regular cooperation and oversight — “quarterly appearances” before Cassidy’s health committee, and ongoing collaboration. Politico
Yesterday, President Trump signed an executive order, as summarized in his post on Truth Social (posted at the top of this Substack essay).
Is HHS Secretary Kennedy required by law to follow President Trump’s executive order, or Senator Cassidy’s edicts?
- This linked document is a Presidential Memorandum (a form of presidential directive) instructing the head of the U.S. Department of Health & Human Services (HHS), i.e. Kennedy, and the head of the Centers for Disease Control and Prevention (CDC) to review international “best practices” and, if appropriate, revise the U.S. childhood vaccine schedule. The White House
- In principle, executive-branch officials (like the HHS Secretary) are under the President’s authority and receive instructions via executive orders, memoranda, and other presidential directives.
- The HHS Secretary does have authority to influence vaccine policy, oversee programs, and guide public-health initiatives. KFF
Thus, from a structural viewpoint, Kennedy is, barring other constraints, expected to act on this directive if it is within the agency’s statutory remit.
Senator Cassidy apparently extracted specific personal commitments from Kennedy during confirmation, about maintaining existing advisory structures, preserving vaccine access, not disrupting current vaccine policy, etc. SciPubHealthLaw
But:
- Those are personal assurances or informal commitments, not formal legal constraints or statutory requirements. There is no legal mechanism that makes them binding in the same way as a law or a duly issued executive directive.
- Once a President issues a valid directive, the Secretary’s duty is to the law and to the President, not to uphold prior personal promises made to a Senator.
In other words, if the new Presidential Memorandum is lawful and within HHS’s statutory authority, Kennedy would have an obligation to follow it, even if it conflicts with what he previously told Cassidy.
Secretary Kennedy and the CDC Director are now released to restructure and align federal vaccine recommendations and policies with norms prevailing in other developed countries.
To provide just three examples illustrating potential impact, many or most of these other countries do not routinely vaccinate for seasonal influenza or for rotavirus infections (sorry, Dr. Paul Offit). And most no longer have recommendations for administering SARS-CoV-2 vaccines.
In Conclusion
This has been a rough few days for the academic/biopharmaceutical vaccine industrial complex. Sacred cows that have been milked annually for decades, and other more recent enormously lucrative mRNA platform-based cash cows, are now at risk of being unceremoniously taken to slaughter.
AS to the CDC ACIP and what just went down, there are stories to tell about the events surrounding these two intensive, extremely stressful days (and the preceding weeks of hard work to get to the votes and voting language) that are best left for private cocktail-party conversations at this point. Suffice to say, this was one of the most stressful and complicated meetings I have ever had to chair.
But we got the job done and lived to fight another day.
This Dire Straits piece (below) pretty much sums up my feelings at this point. I am deeply thankful for all of my Brothers in Arms <Dear Senator Blumenthal – “Brothers in Arms is a metaphor. I am not advocating violence>.
But now, with the President’s new marching orders, we have to turn right around and do it again. Neither rest nor payment for the weary. This is all volunteer.
I suggest that readers may wish to take time to give thanks and prayers for the safety of each and every one of the ACIP members. We are all receiving hate mail and threats after this meeting- which CDC security is now investigating.
We are fools to make war on our Brothers in Arms.











Leave a Reply
You must be logged in to post a comment.