Pathogenic Priming Harnessed Against Cancer!? How a Tree Frog’s Gut Bacterium May Transform ImmunoOncology
December 22 | Posted by mrossol | Medicine, ScienceWow! Science done right brings potentially stunning translational potential for individualized immunotherapy across cancer types. This study reminds me of why I love science.
A new E. americana study is proof-of-concept that a live bacterium, selectively colonizing tumors, can initiate robust tumor-specific immune memory via presentation of novel peptides in an inflammatory context. This is precisely the kind of “danger signal” required for overcoming T cell anergy and immune ignorance toward tumors.
Each year, cancer claims over 600,000 lives in the United States alone. Globally, the toll exceeds 10 million deaths annually, making it the second leading cause of death worldwide. And yet, in the shadow of that sobering figure, it is rare—exceptionally rare—for the public to witness a preclinical experiment so elegant, so disciplined, and so brimming with translational promise as the recent study published in Gut Microbes (2025): “Discovery and characterization of antitumor gut microbiota from amphibians and reptiles: Ewingella americana as a novel therapeutic agent with dual cytotoxic and immunomodulatory properties” (DOI: 10.1080/19490976.2025.2599562).
It is not often that baterial isolates from a frog’s intestine lights a path forward for human cancer therapy. But that is precisely what happened.
The Core Breakthrough
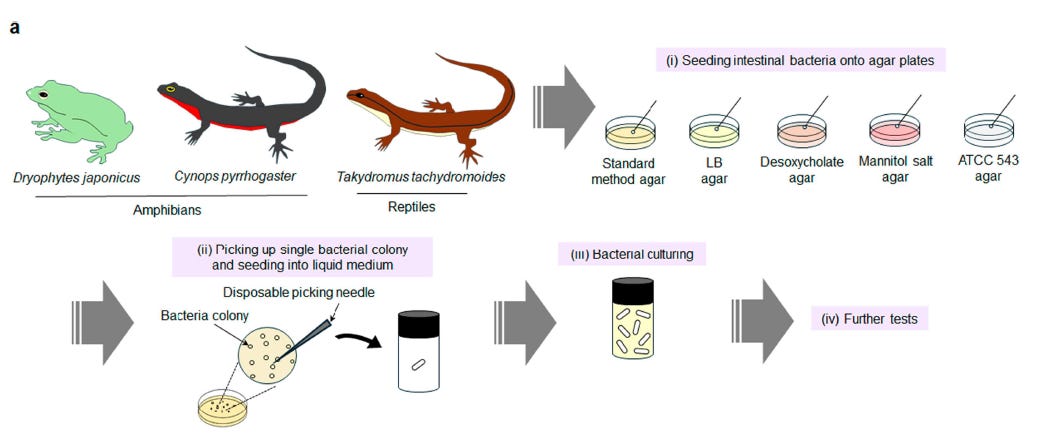
For this discovery, researchers led by Seigo Iwata and Eijiro Miyako from Japan Advanced Institute of Science and Technology (JAIST), specifically at the Graduate School of Advanced Science and Technology, sifted through and carefully studied 45 bacterial isolates drawn from the guts of amphibians and reptiles. They identified Ewingella americana as a standout: a naturally occurring, non-engineered bacterium capable of completely eliminating established colorectal tumors in immunocompetent mice after a single intravenous dose. Mice were injected with Colon-26 carcinoma cells, a syngeneic colorectal tumor line, and were then treated once tumors reached ~200 mm^3. Each candidate bacterial strain was administered via tail vein at a standard dose (200 μL of 5 × 10^9 CFU/mL (colony-forming units/milliliter). Tumors were monitored for 40 days.
Part of Fig 1 from the paper. See the original here. (C) Taylor and Francis.
Nine strains passed biocompatibility and were subjected to in vivo screening. Several strains suppressed tumor growth.
Only E. americana cured tumors outright—and did so with durable, and stunning results. Rechallenge of cured mice resulted in zero tumor recurrence (0/10), while all control mice (10/10) developed tumors. This is immunological memory, not transient suppression.
This is pathogenic priming turning against cancer.
A full experimental timeline spanned 60 days and included biocompatibility screening, tumor implantation, tail-vein delivery of bacterial therapy, tumor volume monitoring, complete blood count (CBC) analysis, histopathology, and tumor rechallenge.
Mechanism of Action: A Dual-Attack Strategy
The therapeutic action of E. americana relies on a two-pronged mechanism that simultaneously targets tumor cells and awakens the host immune system. This is not a blunt-force infection and immune activation like Coley’s toxins; it’s a precision-guided microbial infiltration with a profound immunological twist.
1. Selective Tumor Colonization and Proliferation
E. americana is a facultative anaerobe, meaning it thrives in low-oxygen environments—a hallmark of solid tumors. Once administered intravenously, the bacterium rapidly accumulates inside the tumor microenvironment. Within 24 hours, colony-forming units inside tumors increased by approximately 3,000-fold. Here’s the catch:
. Yet it cleared from systemic circulation within the same time window, as confirmed by serial blood culture assays.
This selective colonization reflects the permissive conditions of hypoxia, immune suppression, necrosis, and altered vascular permeability within tumors. Importantly, the bacterium was not found in normal organs such as the liver, lungs, spleen, or kidneys, underscoring its tumor specificity.
2. Direct Cytotoxicity via Bacterial Effectors
In 3D tumor spheroid co-cultures, E. americana rapidly induced spheroid collapse and cancer cell death in a dose-dependent manner. This cytolysis is mediated by secreted effectors such as hemolysin and exotoxins, which breach cancer cell membranes and disrupt metabolic integrity. Apoptosis was confirmed by both caspase-3 activation and TUNEL staining in tumor tissues.
Even at lower bacterial concentrations, E. americana retained potent cytotoxic effects, suggesting a robust per-cell killing efficiency not reliant on overwhelming bacterial load.
3. Host Immune Recruitment and Memory
The bacterial colonization and subsequent tumor cell death were followed by a sharp rise in immune infiltration. Within 6–24 hours, tumor tissues exhibited:
- Neutrophil accumulation (CXCR4+)
- T cell infiltration (CD3+)
- B cell infiltration (CD19+), and
- Upregulation of key cytokines IFN-γ and TNF-α
These signatures are consistent with rapid and coordinated innate and adaptive immune activation. In effect, the dying tumor becomes an inflammatory training ground for systemic immunity.
A living drug, not a lingering threat
Though E. americana is alive and replicating inside the tumor, it is also susceptible to standard antibiotics and is efficiently cleared by host immunity once its job is done. This built-in self-limiting property, combined with the absence of virulence factors in genomic screens, makes it a compelling candidate for translational development.
It’s an engineered-looking effect, but delivered by nature.
Why This Study Matters
The authors did not shy away from the obvious comparisons. Using parallel treatment arms, they benchmarked E. americana against anti–PD-L1 immunotherapy and liposomal doxorubicin, both administered every other day across four doses at 2.5 mg/kg—a clinically relevant protocol. E. americana outperformed both by a wide margin. It did so with a single dose.
Histopathology and immunohistochemistry told the rest of the story: massive neutrophil infiltration, CD3+ and CD19+ cell mobilization, elevated TNF-α and IFN-γ, and widespread tumor apoptosis via caspase-3 and TUNEL positivity. The bacterium colonized tumors rapidly but cleared from the bloodstream within 24 hours. Organ histology at day 30 was unremarkable. CBCs and chemistry panels showed no deviations from controls.
Even the cytotoxicity assays embraced rigor: 3D spheroid co-cultures showed dose- and time-dependent lysis, with virulence attributed not to pathogenicity but to targeted tumor lysis and immune recruitment. Importantly, all bacterial isolates were administered in their own growth media rather than PBS—a factor noted transparently. Media-only controls will be necessary in future work, but the varied outcomes among strains already argue against media being the primary driver of efficacy.
Clarifying the Ethics
The use of animals in biomedical research remains a point of contention, and rightfully so. But this experiment—designed with statistical efficiency, humane endpoints, and deep relevance to human disease—may help reframe the conversation. Each year, over 10 million people die of cancer. The idea that a previously unknown microbe from the gut of a wild amphibian could trigger complete tumor regression, safely and durably, deserves attention, not censure.
Animal rights concerns are valid, but so is the human toll. Here, the trade-off was made with care, and the payoff was substantial.
BUT WAIT, THERE’S MORE!
In reading the study, it hit me that if bacteria of this species can be modified to express proteins found to be unique to cancers that are killing a patient. Ewingella americana is a facultative anaerobic Gram-negative bacterium that colonizes tumors selectively due to their hypoxic, immune-suppressed microenvironment. Upon intravenous injection, it accumulates inside tumors within hours, proliferating locally while being cleared from the bloodstream in under 24 hours.
This area of research has the immunotherapeutic potential for highly individualized cancer therapies is potentially the turning point in cancer for which we have all been hoping.
Prevention Still Matters
While therapeutic breakthroughs like this give hope to the diagnosed, it remains essential to emphasize prevention. The burden of cancer can be dramatically reduced by limiting exposure to known carcinogens and endocrine disruptors. For a practical guide on environmental and lifestyle carcinogen avoidance, see:
Avoiding cancer is always preferable to curing it. But when cure is necessary, the toolbox must be deep and specific. Cancers evolve.
Unfinished Business, and a Realistic Path Forward
Some tasks remain:
- The therapeutic strain must be sequenced. Currently, only the reference strain genome (ATCC 33852) was used for virulence screening.
- GMP manufacturing protocols must be developed.
- Human dose-scaling requires allometric modeling and cytokine-monitoring protocols.
- Gain-of-function research with bacterial colonies modified and tailored to specific patients (with highly specific epitopes targeting unique, de novo, and tumor-specific immunogenic peptides expressed by a person’s tumors should be greenlit for limited trials in the most hard-to-treat cancer types.
- Combination strategies (e.g., with checkpoint inhibitors or low-dose chemo) may expand safety margins.
- Intratumoral injection, oral delivery, and alternative routes should be explored.
- Regulatory frameworks for live biotherapeutic products (LBPs) must be engaged early.
- Inclusion of orthotopic and metastatic models will enhance translational relevance.
- Vehicle (media-only) control groups should be introduced in future replicates.
- Antibiotic rescue protocols should be validated in parallel.
They are milestones on the translational path.
Here is a regulatory agency- ready proposal:
The National Cancer Institute shoudl greenlight highly contained Phase 1 trials of genetically enhanced tumor-homing bacterial therapies—modified for epitope-specific immune priming—in patients with metastatic, checkpoint-inhibitor–refractory cancers. These trials should focus on safety, clearance kinetics, and immune activation profiles, with response as an exploratory endpoint.
This initiative could then be advanced via NIH/NCI “exceptional responder” pathways or DARPA-style moonshot programs (e.g., PREPARE, AMR).
Final Words
This is not merely a win for science. It is a triumph of method: disciplined screening, ethical design, clear endpoints, and unflinching comparisons to standard-of-care therapies. It’s the kind of paper scientists hope to read, policymakers hope to fund, and patients hope to one day benefit from.
It is also a rare moment when the average citizen—reading past the headlines—might glimpse the delicate precision, biological imagination, and intellectual clarity required to move the frontier forward. A moment where an amphibian’s gut offered up a living therapeutic that may yet change the arc of human cancer treatment.
The public rarely gets to see science this clean.
Let’s make sure they see truly remarkable and promising studies like this one in the future.
What are your thoughts? Is this type of research worth the ethical burden? Should gain-of-function research be conducted on bacteria that could help our immune systems save our lives? Leave a comment and share.
80% OFF – LIVE COURSE W/DR. LYONS-WEILER
THE BIOLOGY OF CANCER
SIGN UP TODAY FOR A JANUARY 2025 GROUP – MEET WEEKLY W/PEERS





Leave a Reply
You must be logged in to post a comment.