The Schedule Is the Signal: Why the January 2026 Vaccine Policy Reset Was Necessary, Not Radical
January 6 | Posted by mrossol | CDC NIH, Critical Thinking, Transparency[non], VaccineCDC Cuts Total Doses in alignment with the rest of most of Western Civilization. This is long overdue and the experience will prove it was the right decision/direction. mrossol
Source: The Schedule Is the Signal: Why the January 2026 Vaccine Policy Reset Was Necessary, Not Radical
James Lyons-Weiler, PHD
Popular Rationalism
The CDC’s January 2026 childhood vaccine schedule realignment is not a retreat from science—it is its restoration. By aligning the U.S. with international norms, reclassifying low-benefit vaccines, and preserving universal access, the policy reasserts informed consent, parsimony, and scientific integrity as central to public health. This editorial evaluates the evidence, clarifies common misinterpretations, and outlines the stakes of institutional credibility in the era of collapsing trust.
Ending the Era of Maximalism
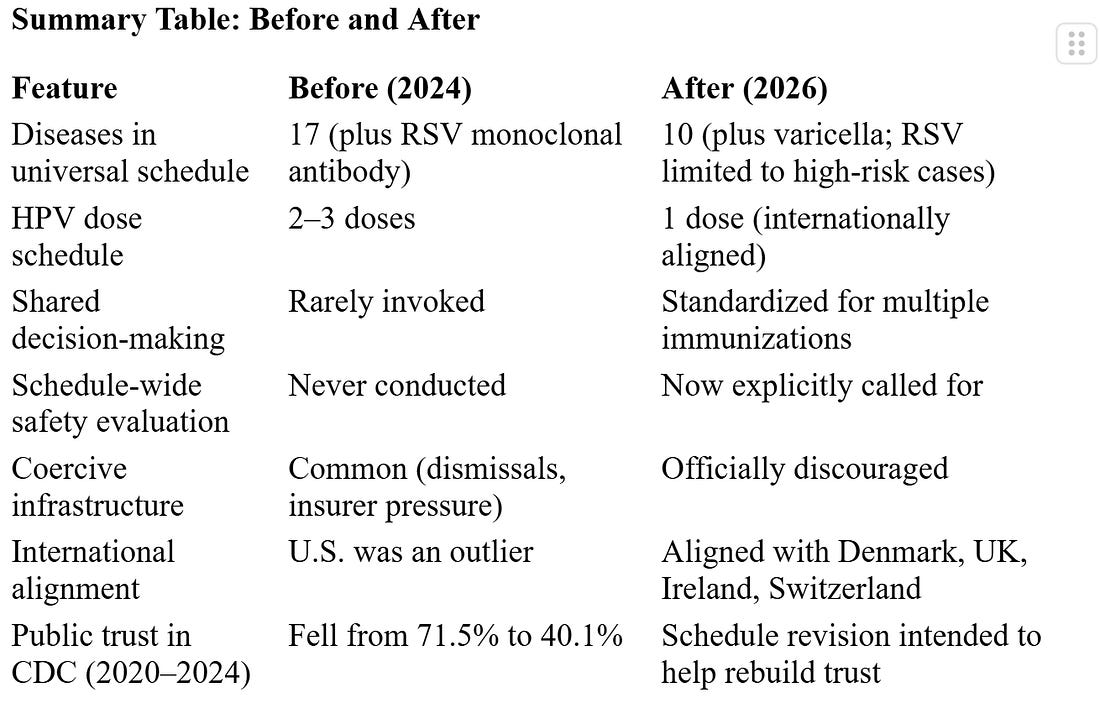
In January 2026, the CDC issued a long-overdue correction to the American childhood vaccine schedule. Despite headlines framing this move as a rollback or retreat, not a single vaccine was removed from access or coverage. The change was not reductive—it was clarifying. It replaced one-size-fits-all mandates with a proportional, transparent structure based on international norms, current evidence, and a sobering admission of what science does not yet know. This was not a political maneuver. It was a governance correction, rooted in the principles of informed consent and institutional legitimacy.
The real story is not what was removed, but what was realigned—and why. The revised architecture reflects a basic truth: trust cannot be coerced. It must be earned. That is the starting point of science. And the endpoint of policy.
The CDC Recognizes Its Schedule as a Coercive Instrument
For decades, the CDC’s “routine recommendation” has operated less as guidance and more as soft mandate. Once a vaccine was recommended for all children, it cascaded through state school-entry requirements, insurance policies, quality metric scoring, and pediatrician compliance programs. Families who opted out often faced dismissal from care. Physicians faced insurer incentives tied to vaccination quotas. In this ecosystem, choice was technically permitted—but penalized.
The CDC’s own assessment acknowledges this explicitly: “Instead of implementing vaccination mandates, most peer nations maintain high childhood vaccination rates through public trust and education” (CDC, 2026, p.3). The updated policy aims to dismantle this coercive scaffolding—not by withdrawing vaccines, but by restoring clarity to what is essential, what is conditional, and what is contextual.
Comparative Overreach: America as an Outlier
The United States was not just a global leader in pediatric vaccination. It was a statistical outlier. According to the CDC’s comparative review (2026, Table 2), the U.S. schedule in 2024 recommended vaccines against 17 diseases, requiring 84 to 88 total doses delivered across 57 to 71 injections. By contrast:
- Denmark covers 10 diseases with 30 doses and only 11 injections.
- UK uses fewer doses but retains near-universal MMR uptake.
- Canada varies by province but aligns closely with European practice.
Importantly, many peer nations refrain from recommending routine use of hepatitis A, influenza, meningococcal B, and rotavirus for all children. These are not poor or negligent countries. They are scientifically robust, and they achieve high uptake by preserving credibility, not enforcing compliance.
The report introduces the ethical principle of clinical equipoise—the acknowledgment of uncertainty in the face of professional disagreement. When peer nations with equivalent disease burdens and health infrastructures diverge in recommendations, it signals unresolved evidence gaps, not ignorance.
Trust Collapse and Its Operational Consequences
Trust in U.S. health authorities fell precipitously between 2020 and 2024—from 71.5% to 40.1% (CDC, p.3). This collapse had measurable consequences. Uptake of the MMR vaccine, one of the most effective vaccines in the consensus schedule, dropped from 95.2% to 92.7% nationally. Sixteen states fell below the 90% threshold, increasing the risk of outbreaks.
Indeed, in 2025, the U.S. experienced 49 measles outbreaks—88% of the 2,065 reported cases were outbreak-associated (CDC, 2026). This wasn’t due to vaccine rejection. It was due to trust rejection. The report directly links trust erosion to coercive COVID-era policies, including mask mandates, school closures, disregard for natural immunity, and overstated claims about sterilizing immunity. The CDC writes, “The distrust of public health agencies during the pandemic has spilled over to other recommendations, including those with respect to vaccines” (p.3).
This trust decay wasn’t isolated. Countries like Denmark explicitly warned against adding low-benefit vaccines to their schedules, citing risks of degrading public confidence. Their prediction came true here. The U.S. attempted to do more—and got less.
Schedule-Level Science: Gaps Finally Acknowledged
The most important admission in the report may be this: “The effects of the overall schedule have never been fully evaluated” (CDC, p.12). That sentence should haunt anyone who defends the status quo. Despite decades of schedule expansion, there has been no comprehensive evaluation of the long-term safety, synergy, or cumulative immunologic impact of the entire pediatric vaccine regimen.
While individual vaccines like MMR, Hib, and IPV have robust pre-licensure data, many others were approved without large-scale placebo-controlled trials. Post-marketing systems such as VAERS, VSD, and BEST have identified acute risks—e.g., intussusception with rotavirus, febrile seizures with MMRV, myocarditis with mRNA vaccines—but are underpowered for delayed or systemic effects.
A 2023 VSD study found a dose-dependent association between cumulative aluminum exposure from vaccines and persistent asthma (HR = 2.0) (Daley et al., Academic Pediatrics, 2023). This is not conclusive proof of harm—but it is definitive proof of the need to study schedule-level interactions.
The CDC now calls for exactly that: randomized timing trials, long-term cohort studies comparing health outcomes across exposure strata, and formal evaluation of interaction effects, adjuvant loads, and timing differentials.
A New Ethical Architecture
The revised schedule distinguishes three recommendation types:
1. Recommended for all children — reserved for vaccines with demonstrated benefit across the population and international consensus.
2. High-risk group recommendations — for children with defined medical or exposure risks.
3. Shared clinical decision-making — for vaccines where the population-level benefit is uncertain, or where individual risk–benefit may vary.
This framework already exists in CDC language, but it had been underutilized and obscured by the dominance of routine recommendations. The new policy makes it operational.
Crucially, no vaccines are removed from coverage. The document reiterates: “All immunizations recommended by the CDC at the end of 2025—and covered by insurance at that time—should remain covered without cost sharing” (CDC, p.3). Denmark, the UK, and Switzerland use similar stratified systems. The U.S. has now caught up—not by doing less, but by doing what works.
HPV One-Dose: An Evidence-Based Pivot
The decision to shift from two doses of HPV vaccine to one is a model for evidence-responsive policy. The CDC cites multiple studies demonstrating non-inferiority of a single dose:
– Kreimer et al., NEJM 2025
– Watson-Jones et al., Lancet Global Health 2025
– Basu et al., Lancet Oncology 2021
Peer nations including the UK, Ireland, Australia, and Canada had already adopted this strategy. One dose achieves near-identical protection against vaccine-targeted HPVs with lower burden and fewer adverse events. The CDC’s alignment here is not a retreat—it’s a data-driven upgrade.
Refined “Recommended for All” List
The CDC now limits routine universal recommendations to vaccines with:
– Strong international consensus
– High demonstrated public health value
– Well-characterized safety and efficacy profiles.
These are:
– Measles, mumps, rubella (MMR)
– Diphtheria, tetanus, pertussis (DTaP/Tdap)
– Polio (IPV) – Haemophilus influenzae type B (Hib)
– Pneumococcal conjugate (PCV)
– Human papillomavirus (HPV), now reduced to a single-dose schedule
– Varicella (chickenpox), retained due to U.S.-specific epidemiology
Many parents have questions about the efficacy of the measles and mumps portions of the MMR given that asymptomatic transmission of measles is an established but little-discussed fact, and before COVID-19, mumps outbreaks in fully vaccinated schools in the US was well-documented.
What changed: HPV was reduced from 2–3 doses to 1. Several vaccines previously listed as universal are now reclassified. The new universal list more closely mirrors countries like Denmark, the UK, and Ireland.
Reclassification of Non-Consensus Vaccines
Vaccines such as:
– Hepatitis A
– Hepatitis B (birth dose only if mother is HBsAg-negative)
– Rotavirus
– Influenza
– COVID-19
– Meningococcal B and ACWY
– RSV monoclonal antibody (not a vaccine)
have all been moved to either:
– High-risk group recommendations (e.g., Hep A for travelers, Hep B for infants of positive/unknown mothers)
– or Shared clinical decision-making pathways
This model mirrors European governance practices, where vaccines with uncertain population-wide benefit are discussed individually between provider and parent/guardian.
What changed: These vaccines are no longer recommended for universal administration but remain fully covered and available to all families through Medicaid, CHIP, VFC, and private insurance.
Policy Emphasis on Schedule-Level Science
For the first time, the CDC acknowledges:
– The full schedule has never been rigorously studied for cumulative, synergistic, or long-term effects
– Many vaccines were approved without randomized placebo-controlled trials in children
– Post-licensure surveillance (e.g., VAERS, VSD) is underpowered to detect long-latency effects or rare but serious chronic sequelae
The CDC now explicitly calls for:
– Randomized trials using timing-based designs
– Long-term cohort studies comparing vaccinated vs unvaccinated children
– Safety studies on combined vaccine administration, adjuvants, and spacing.
This is a seachange: Scientific uncertainty is now acknowledged and embedded into the policy framework, triggering a new research mandate.
Elimination of Implicit Coercion via Schedule
While the policy does not change state-level school mandates, it removes the federal “routine” label from lower-priority vaccines, reducing pressure on providers to dismiss non-compliant families or tie insurer bonuses to rigid adherence.
In its place: a structured, choice-respecting pathway that centers parental informed consent.
What changed: The policy restores consent as a governing principle, removes schedule inflation, and distinguishes between access and recommendation.
This is a systemic reform, not a minor tweak. The policy shift restores proportionality, science-based prioritization, and institutional humility—while safeguarding coverage and access. It is a reassertion of legitimacy in the aftermath of a trust crisis.
What the Policy Rejects
This policy formally rejects several assumptions that had ossified into doctrine:
– That more vaccines necessarily equal better health.
– That mandates are required to ensure compliance.
– That high-volume schedules are scientifically complete.
– That dissent is misinformation.
– That informed consent is a formality, not a right.
The CDC explicitly names coercion as a failed tool and calls for its replacement with personalized, risk-aligned care.
What the Policy Preserves and Strengthens
This is not a deregulation agenda. It is a realignment. The policy preserves:
– Universal access to all covered vaccines.
– Full coverage under Medicaid, CHIP, and VFC.
– Trust-based compliance mechanisms.
– Ethical clarity: recommendations reflect both evidence and respect for autonomy.
– Institutional epistemic humility: public health must now justify, not presume.
The result? Less friction, more uptake—of the right vaccines, in the right populations, for the right reasons.
Anticipating and Answering the Critics
No, the liability protections were not removed. This policy does not increase vaccine risk—it increases institutional honesty.
No, measles will not surge because of this schedule. MMR remains fully recommended. The drop in uptake happened under maximalist policy.
No, international comparison is not cherry-picking. It is the standard for identifying clinical equipoise. Denmark, Germany, Ireland, and Switzerland offer leaner schedules, fewer mandates, and stronger vaccine trust.
Those who call this “anti-science” misunderstand science. This is science doing what it must: confronting uncertainty, not denying it.
The Schedule Is the Signal
The CDC’s January 2026 reform is not the dismantling of public health. It is its restoration. Trust cannot be coerced. Compliance must be earned. And scientific legitimacy must be updated to reflect both what we know—and what we still don’t.
The vaccine schedule is not just a list. It is a social contract. And for the first time in decades, it has been revised to reflect mutual respect, rather than managerial force.
The signal has changed. And for the health of children and the credibility of science, that is exactly what was needed.
Citations
- Centers for Disease Control and Prevention. Assessment of the U.S. Childhood and Adolescent Immunization Schedule Compared to Other Countries. January 2, 2026. Accessed January 5, 2026. https://www.hhs.gov/sites/default/files/assessment-of-the-us-childhood-and-adolescent-immunization-schedule-compared-to-other-countries.pdf
- Centers for Disease Control and Prevention. Child and Adolescent Immunization Schedule by Age. Updated August 7, 2025. https://www.cdc.gov/vaccines/hcp/imz-schedules/child-adolescent-age.html
- Centers for Disease Control and Prevention. Measles Cases and Outbreaks in the U.S. Accessed January 5, 2026. https://www.cdc.gov/measles/cases-outbreaks.html
- Daley MF, Reifler LM, Glanz JM, et al. Association between aluminum exposure from vaccines before age 24 months and persistent asthma at age 24 to 59 months. Academic Pediatrics. 2023;23(1):37-46. https://pubmed.ncbi.nlm.nih.gov/36180331/
- Kreimer AR, et al. Single-Dose Human Papillomavirus Vaccination: State of the Science. J Natl Cancer Inst Monogr. 2024;2024(59):1-27. https://pmc.ncbi.nlm.nih.gov/articles/PMC12104495/
- Centers for Disease Control and Prevention. Shared Clinical Decision-Making Vaccine Recommendations. Accessed January 5, 2026. https://www.cdc.gov/acip/vaccine-recommendations/shared-clinical-decision-making.html
- Reuters. US cuts broad recommendation for four childhood vaccines. January 5, 2026. https://www.reuters.com/business/healthcare-pharmaceuticals/us-revises-childhood-vaccine-schedule-recommend-fewer-shots-2026-01-05/
- Washington Post. U.S. overhauls childhood vaccine schedule, recommends fewer shots. January 5, 2026. https://www.washingtonpost.com/health/2026/01/05/childhood-vaccine-immunization-schedule-overhaul/
- Associated Press. US cuts the number of vaccines recommended for every child. January 5, 2026. https://apnews.com/article/9b8df9e2767c1261aaac4e2331e77fa3
- European Centre for Disease Prevention and Control. Vaccine Schedules in All Countries in the EU/EEA. Accessed January 5, 2026. https://vaccineschedule.ecdc.europa.eu
- Statens Serum Institut (Denmark). Childhood Vaccination Programme. Accessed January 5, 2026. https://www.ssi.dk/vaccination/born
- Government of Canada. Canadian Immunization Guide. Updated July 2025. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide.html
- Health Service Executive (Ireland). Immunisation Schedule. 2025. https://www.hse.ie/eng/health/immunisation/pubinfo/pcischedule/immschedule.html
- Levison LS, Thomsen RW, Andersen H. Guillain-Barré syndrome following influenza vaccination: A 15-year nationwide population-based case-control study. European Journal of Neurology. 2022;29(11):3389-3394. https://doi.org/10.1111/ene.15534
- Sarkanen TO, Alakuijala AP, Dauvilliers YA, et al. Incidence of narcolepsy after H1N1 influenza and vaccinations: systematic review and meta-analysis. Sleep Medicine Reviews. 2018;38:177-186. https://doi.org/10.1016/j.smrv.2017.05.006
- Jefferson T, Rivetti A, Di Pietrantonj C, et al. Vaccines for preventing influenza in healthy children. Cochrane Database of Systematic Reviews. 2018;2(2):CD004879. https://doi.org/10.1002/14651858.CD004879.pub5
- Institute of Medicine. The Childhood Immunization Schedule and Safety: Stakeholder Concerns, Scientific Evidence, and Future Studies. Washington, DC: National Academies Press; 2013. https://doi.org/10.17226/13563
- CIDRAP. Single HPV vaccine dose matches protection of 2-dose regimen, new trial shows. October 31, 2023. https://www.cidrap.umn.edu/human-papillomavirus-hpv/single-hpv-vaccine-dose-matches-protection-2-dose-regimen-new-trial-shows
Thank you for being a subscriber to Popular Rationalism. For the full experience, become a paying subscriber. And check out our awesome, in-depth, live full semester courses at IPAK-EDU. Hope to see you in class!





Leave a Reply
You must be logged in to post a comment.