What does the annual covid19 shot mean for the annual flu shot ?
September 2 | Posted by mrossol | Medicine, Prasad, Science, VaccineSource: (1) What does the annual covid19 shot mean for the annual flu shot ?
Yesterday I described the situation with the COVID-19 vaccines. Specifically, the administration has marched forward with an annual vaccination in the absence of any clinical data. They are pushing the dose in young people, who are at risk of myocarditis, and have staggeringly little to gain from continued boosting. Two senior FDA officials resigned over this idea (boosters for all).
The current US guidance is counter to guidance from the United Kingdom to restrict the use largely to the elderly. Neither group has good data to justify their choice, but the US decision is particularly illogical and heavy-handed.
How does the flu vaccine compare? Each year a new dose is debuted without randomized data at the time of approval and, often, even thereafter. Is the flu vaccine like the COVID shot?
First, let’s consider ways they are different
- Annual covid shots are far more immunogenic than flu shots. People feel worse after the dose. More people take a day off work. Rates of pain and rigors and fevers are greater. This matters for public policy considerations. The days of life lost (spent in bed) count against this campaign.
- Annual covid shots are different because the age risk gradient is far steeper for covid-19 than flu and the upper bound benefit to younger people is more debatable. We also know less about the future risk of COVID to a 55 yo than the future risk of flu to a 55 yo.
- Annual covid shots have one serious safety concern— myocarditis, and likely excess in cardiovascular mortality among young men. This and other safety concerns— tinnitus etc.— have been inadequately studied as the current climate is hostile to vaccine safety research.
- In contrast with flu shots, COVID shots have a pending, and postponed post marketing commitment to generate data on subclinical (troponin leak) myocarditis in young men. The FDA and the company seem disinterested in generating these data.
- Gruber and Krause never resigned over flu shots, and no president leaned on FDA to grant EUA.
Now let’s consider how they are similar
- From the 2018 Cochrane review for healthy adults “Inactivated vaccines can reduce the proportion of healthy adults (including pregnant women) who have influenza and ILI, but their impact is modest. We are uncertain about the effects of inactivated vaccines on working days lost or serious complications of influenza during influenza season”
- From the 2018 update on kids, “In children aged between 3 and 16 years, live influenza vaccines probably reduce influenza (moderate‐certainty evidence) and may reduce ILI (low‐certainty evidence) over a single influenza season. In this population, inactivated vaccines also reduce influenza (high‐certainty evidence) and may reduce ILI (low‐certainty evidence). For both vaccine types, the absolute reduction in influenza and ILI varied considerably across the study populations, making it difficult to predict how these findings translate to different settings. We found very few randomised controlled trials in children under two years of age.
- For the elderly, “Older adults receiving the influenza vaccine may have a lower risk of influenza (from 6% to 2.4%), and probably have a lower risk of ILI compared with those who do not receive a vaccination over the course of a single influenza season (from 6% to 3.5%). We are uncertain how big a difference these vaccines will make across different seasons. Very few deaths occurred, and no data on hospitalisation were reported.”
- In other words— flu shots WOULD benefit from more randomization. We know nearly nothing about more important endpoints such as hospitalization or transmission dynamics. These can include trials early in the season— and can occur alongside implementation. Pragmatic design where tens of thousands of people are continually randomized till endpoint is met, or even…
- Randomized trials of deployment similar to this proposal.
- Challenge trials— aka Randomized studies— could also be performed.
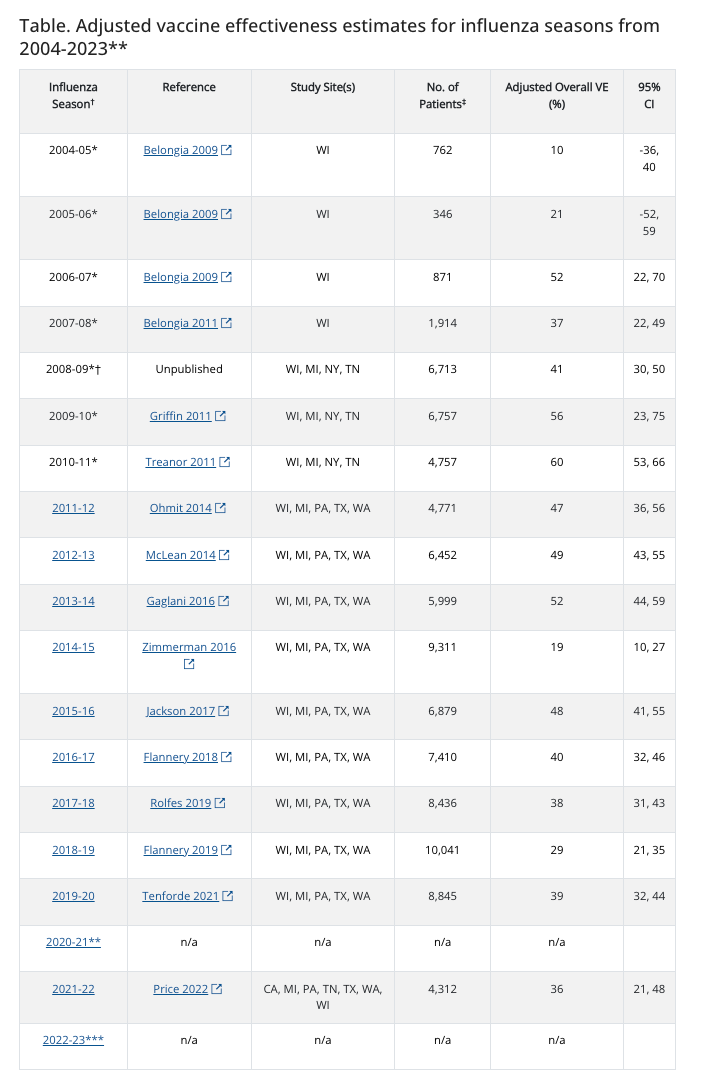
- In the absence of randomized trials, we rely on test negative case control studies to assess flu vaccine efficacy, which is the CDC’s preferred method. In my opinion, this method exaggerates effectiveness over RCTs, but to prove this claim (or the counterclaim— that it is accurate) one has to compare this method to concurrent RCTs, which has not been done. Here is the CDC’s VE from flu shots. Ranging from 10% to 60%.
These numbers are low, and this suggests immediately that each season a multi-arm RCT can be conduct of different vaccine formations and control arm, and the winner could be advanced. The entire idea of one shot prior to the season may be suboptimal— even if flu shots work, why not have several candidates and pick the best one?
- Putting this all together, I agree with the attached essay by Doshi. I think there is little justification for mandatory HCW vaccination. I do not push influenza vaccines in young, healthy people. Vaccinating children 6mo-2yo has particularly low credibility data.
Dozens of RCTs are needed, and in the absence, Doshi’s concerns— articulated in the BMJ 10 years ago— are salient. Many doctors I know decline the annual flu shot if that is an option. I suspect this is under discussed due to the nature of the subject. I attach the full PDF for those interested.
|
||
You’re currently a free subscriber to Vinay Prasad’s Observations and Thoughts. For the full experience, upgrade your subscription.







Leave a Reply
You must be logged in to post a comment.