HHS Abdicates on Pediatric Dose Limit in Vaccines, Defers instead to the Peer-Reviewed Literature. That’s Now IPAK.
April 8 | Posted by mrossol | FDA, Medicine, Science, VaccineI include because I believe that we all need to improve our understanding of benefits and also the risks of vaccines. No simply the disease agent the vaccine is designed to protect against, but all the other “inert ingredients”, the fillers, the chemicals which improve the vaccine shelf-life, or improve its effectiveness. mrossol
“For the 850 mcg dose FDA claims is safe using the wrong types of aluminum, the wrong type of mammal, the wrong route of exposure, and a single cherry-picked, misinterpreted study, a person would have to weigh 374 pounds to avoid exceeding the FDA’s aluminum safe level for intravenous exposure.”
In 2019, the Informed Consent Action Network (ICAN) sent US HHS requested, via FOIA:
“Copies of any human or animal studies involving the subcutaneous or intramuscular injection of aluminum adjuvant relied upon by the CDC to establish the safety of injecting infants and children with aluminum hydroxide, aluminum phosphate or amorphous aluminum hydroxyphosphate sulfate.”
US HHS with the following:
“A search of our records failed to reveal any documents pertaining to your request. Specifically, CDC’s Immunization Safety Office (ISO) states that ‘[t]his request is outside of ISO purview and should be referred to the U.S. Food and Drug Administration.[FDA]’ FDA’s contact information is as follows:”
An appeal was filed, and ICAN was ultimately told by HHS to check the peer-reviewed literature:
HHS suggested they use “the following NIH webpages to possibly find publicly available information relevant to your request”. That request included Pubmed.
By the time HHS had replied, IPAK had already gone over the entire literature and published the full story of how 850 mcg of aluminum was determined that an 850 mcg dose of aluminum hydroxide per dose was “safe”.
We had provided the world with the first pediatric dose limit (PDL) of exposure to aluminum hydroxide in vaccines based on standard toxicological consideration of body weight. This is particularly important since the same compound is used to routinely and reliably induce autoimmune conditions in rats and mice at doses per body weight that overlap those human infants experience during early to mid-infancy. However, the FDA’s 850 mcg per dose was not based on body weight.
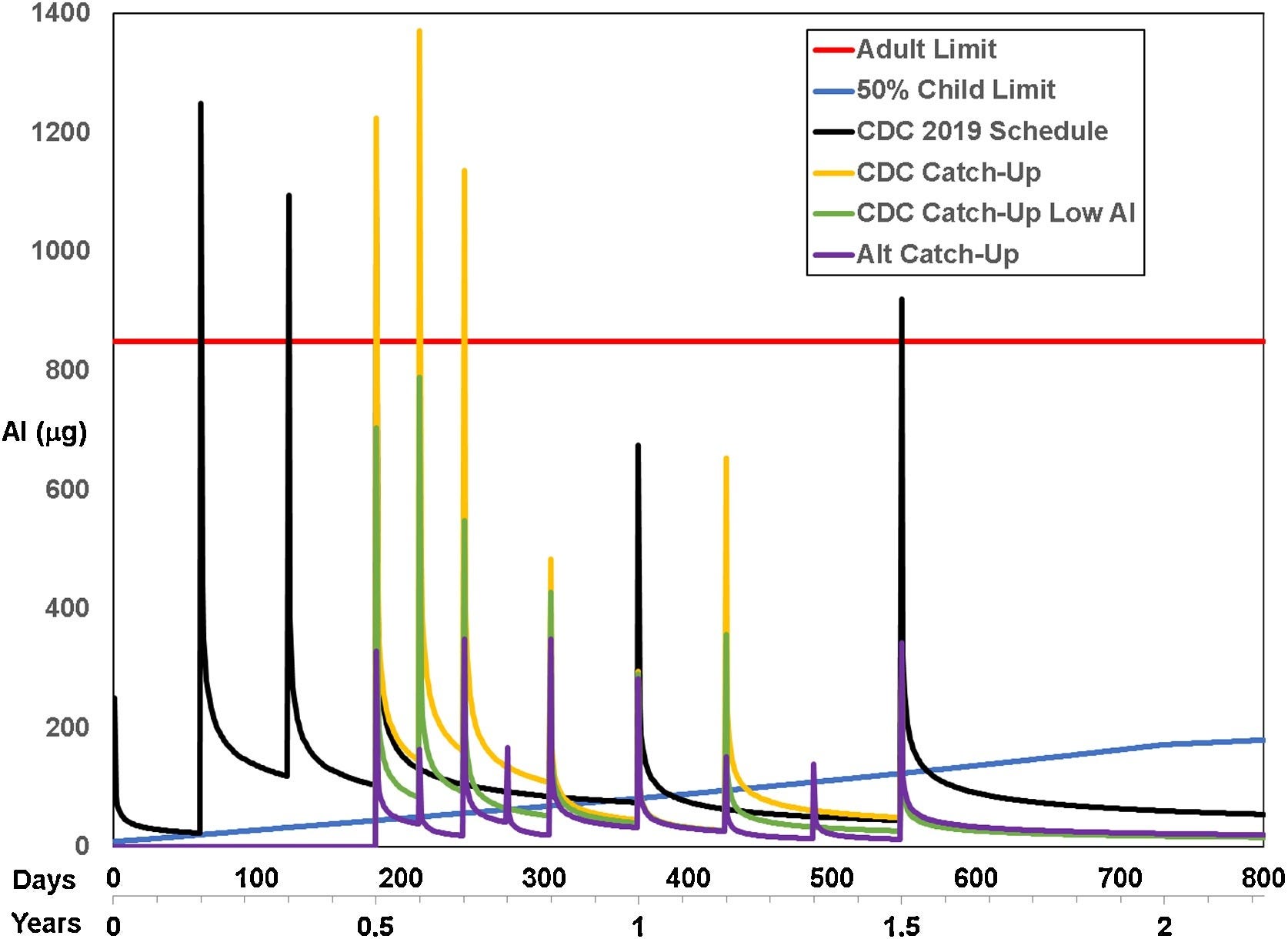
See the blue line in this image? That’s the body-weight adjusted PDL we published (also based on consideration to kidney development up to two years) of the 50 %tile body weight of children at each age. The orange line is FDA’s 850 mcg so-called ‘safe’ dose of of 850 mcg per vaccine. The lines with the peaks correspond to uptake and clearance under various schedules for the various schedules studied.
Clearly, aluminum exposure does not only have acute toxicity (peaks); it also has chronic exposure – wherever the clearance lines exceed the blue PDL line, infants and toddlers are experiencing the health effects of whole-body aluminum toxicity, including risks of autoimmunity and food allergies.
This particular result is from our latest published study. We now have three studies examining the PDL and its use in combination with injection-based clearance models based on subcutaneous injection of radioactive aluminum hydroxide (previously published, IPAK never injected anyone), and we published that
- FDA relied on oral forms of aluminum in adult mice to estimate safe levels of aluminum in humans, and relied mostly on one study by Golub et al that, contrary to their report, DID show adverse effects in the mice tested (cyclic feeding),
- Oral forms are only absorbed at a rate of 0.03%, whereas 100% of aluminum injected in vaccines have to be dealt with by the body.
- Infants on day 1 of life receive, in the HepB vaccine, over 11 times more aluminum hydroxide per body than an adult would be allowed to receive from a vaccine,
- Infants are in whole body aluminum toxicity 100% of the days in the first year of life.
- Aluminum toxicity in the pediatric schedule leads to chronic aluminum whole-body toxicity.
Even simple math using FDA’s only per-body weight claim shows that pediatric vaccinates are not safe. The FDA regulation (21 CFR 201.323) on aluminum exposure from parenteral drug products, including those used in total parenteral nutrition (TPN), requires that the amount of aluminum present at the time of administration be no greater than:
“For Large Volume Parenterals (LVPs) and Small Volume Parenterals (SVPs): The product’s label must state that the maximum level of aluminum does not exceed 25 micrograms per liter (µg/L).”
Additionally, specific to parenteral products used in TPN:
Warning Label for Products with Aluminum Content Exceeding 5 Micrograms per Liter: The regulation mandates a warning label on products that could result in a daily aluminum intake exceeding 5 micrograms per kilogram (µg/kg) of body weight per day.
Therefore, while the regulation asserts a limit of 5 µg/kg/day of aluminum from parenteral nutrition sources for humans. This is an important consideration when evaluating the overall exposure to aluminum, particularly in vulnerable groups receiving TPN.
Given FDA’s limit, for a 2.2 kg newborn, based on the limit of 5 micrograms of aluminum per kilogram per day, the total allowable daily exposure would only be 11 micrograms of aluminum per day.
HepB vaccine has 250 mcg. See the problem here? Children are to 50 kg until around age 11 or 12. For the 850 mcg dose FDA claims is safe using the wrong types of aluminum, the wrong type of mammal, the wrong route of exposure, and a single cherry-picked, misinterpreted study, a person would have to weigh 374 pounds to avoid exceeding the FDA’s aluminum safe level for intravenous exposure.
We are now writing up the results of our study of various vaccine schedules around the world and have some shocking results.
FactCheck.org Gets So Much Wrong. Where to Start?
This so-called Fact Check opinion blog article, published on April 5,
claims to be “Debunking Viral Claims”. They have done no such thing – not even close.
They commit the “other sources contribute more” fallacy:
“The researchers found that during infancy, the aluminum from vaccines at most might contribute twice the amount that the body absorbs from dietary sources, taking into account that the vast majority of aluminum in food or drinks is never absorbed into the body. Other research has indicated that overall, vaccines contribute a negligible amount of aluminum to children’s total exposure.”
Imagine if someone were so misinformed about toxicology that they argued that lead in Flint, Michigan’s water was not a concern because lead was also in the soil and paint of old homes. That person would clearly be making a logical error; the other exposures make the exposure from water MORE, not LESS of a concern.
Similarly, aluminum in water, breast milk, and food should increase, not decrease, concern over aluminum in vaccines. The public should be advised not to fall for this cheap logical gambit.
Their claims of more aluminum from other sources are also mathematically incorrect given routes of absorption: we absorb only 0.03% of aluminum ingested, and thus only 0.03% of ingested aluminum contributes to the body burden. By comparison, 100% of injected aluminum contributes to body burden and must be dealt with by our bodies.
IPAK also published this is our peer-reviewed study
See the source here; it is Supplementary to Lyons-Weiler et al. (2020a).
The opinion blog article author then wrote:
“One study, published in 2017 in Academic Pediatrics, took blood and hair samples from 85 healthy children between 9 and 13 months of age. The researchers did not find a correlation between the vaccines the children had received and the amount of aluminum in their hair or blood, either when looking at total vaccine history or the vaccines they’d gotten the day of testing. This is in keeping with results of a smaller study of 2-month-old preterm infants, which also didn’t find a relationship between vaccination and blood aluminum levels.”
The pharmacodynamics of aluminum predetermined these results: One must perform partial chelation prior to testing for aluminum in the blood (this is called a challenge test); any physician can order such a test at Great Plains Testing (now Mosaic Testing). Hair samples do not track aluminum’s whole-body toxicity. It’s time for an objective study of whole-body aluminum body burden using challenge chelation testing.
Given that the author of the opinion blog article posing as a “Fact Checker” knows that HHS referred ICAN to the published literature, one would expect that she might have done some legwork and found the peer-reviewed literature and these peer-reviewed studies published by IPAK (below). No peer-reviewed studies have found our results incorrect; an attempt to retract one of our studies failed.
To help our, here are our published studies on this so far:
Abstract. FDA regulations require safety testing of constituent ingredients in drugs (21 CFR 610.15). With the exception of extraneous proteins, no component safety testing is required for vaccines or vaccine schedules. The dosing of aluminum in vaccines is based on the production of antibody titers, not safety science. Here we estimate a Pediatric Dose Limit that considers body weight. We identify several serious historical missteps in past analyses of provisional safe levels of aluminum in vaccines, and provide updates relevant to infant aluminum exposure in the pediatric schedule considering pediatric body weight. When aluminum doses are estimated from Federal Regulatory Code given body weight, exposure from the current vaccine schedule are found to exceed our estimate of a weight-corrected Pediatric Dose Limit. Our calculations show that the levels of aluminum suggested by the currently used limits place infants at risk of acute, repeated, and possibly chronic exposures of toxic levels of aluminum in modern vaccine schedules. Individual adult exposures are on par with Provisional Tolerable Weekly Intake “limits”, but some individuals may be aluminum intolerant due to genetics or previous exposures. Vaccination in neonates and low birth-weight infants must be re-assessed; other implications for the use of aluminum-containing vaccines, and additional limitations in our understanding of neurotoxicity and safety levels of aluminum in biologics are discussed.
Abstract. Like the mechanisms of action as adjuvants, the pharmacodynamics of injected forms of aluminum commonly used in vaccines are not well-characterized, particularly with respect to how differences in schedules impact accumulation and how factors such as genetics and environmental influences on detoxification influence clearance. Previous modeling efforts are based on very little empirical data, with the model by Priest based on whole-body clearance rates estimated from a study involving a single human subject. In this analysis, we explore the expected acute exposures and longer-term whole-body accumulation/clearance across three vaccination schedules: the current US Centers for Disease Control and Prevention (CDC) schedule, the current CDC schedule using low aluminum or no aluminum vaccines, and Dr. Paul Thomas’ “Vaccine Friendly Plan” schedule. We then study the effects of an implicitassumption of the Priest model on whether clearance dynamics from successive doses are influenced by the current level of aluminum or modeled by the assumption that a new dose has its own whole-body dynamics “reset” on the day of injection. We model two additional factors: variation (deficiency) in aluminum detoxification, and a factor added to the Priest equation to model the potential impact of aluminum itself on cellular and whole-body detoxification. These explorations are compared to a previously estimated pediatric dose limit (PDL) of whole-body aluminum exposure and provide a new statistic: %alumTox, the (expected) percentage of days (or weeks) an infant is in aluminum toxicity, reflecting chronic toxicity. We show that among three schedules, the CDC schedule results in the highest %alumTox regardless of model assumptions, and the Vaccine Friendly Plan schedule, which avoids >1 ACV per office visit results in the lowest (expected) %alumTox. These results are conservative, as the MSL is derived from data used by FDA to estimate safety of aluminum in adult humans. These results demonstrate high potential utility of modeling variation in patient responses to aluminum. More empirical data from individuals who are suspected of being intolerant of aluminum from vaccines, evidenced by high aluminum retention, neurodevelopmental disorders and/or a myriad of chronic illnesses would help answer questions on whether the model predictions can be used to estimate parameter values tied to genetic factors including genomic sequence variation and family history of chronic illnesses tied to aluminum exposure.
Abstract: BACKGROUND: The COVID-19 pandemic has placed significant stressors on the medical community and on the general public. Part of this includes patients skipping well-child visits to reduce risk of exposure to SARS-CoV-2 virus. Published estimates of the duration of whole-body aluminum (Al) toxicity from vaccines in infants from birth to six months indicate that CDC’s recommended vaccination schedule leads to unacceptably long periods of time in which infants are in aluminum toxicity (as measured by %AlumTox).
METHODS: We utilize these established clearance and accumulation models to calculate expected per-body-weight whole-body toxicity of aluminum from vaccines considering for children of all ages under CDC’s Catch-Up schedule from birth to ten years, assuming social distancing for 6 months. Our updated Pediatric Dose Limit (PDL) model assumes a linear improvement in renal function from birth to two years.
RESULTS: Our results indicate that due diligence in considering alternative spacing and use of non-aluminum containing vaccines when possible will reduce whole body toxicity and may reduce risk of morbidity associated with exposure to aluminum.
CONCLUSIONS: While reduction or elimination of aluminum exposure from all sources is always a good idea, our results indicate that careful consideration of expected aluminum exposures during regular and Catch-Up vaccination is found to be especially important for infants and children below 2 years of age. We urge caution in the mass re-starting of vaccination under CDC’s Catch-Up schedule for children under 12 months and offer alternative strategies to minimize per-day/week/month exposure to aluminum hydroxide following the COVID-19 period of isolation.
Thank you for being a subscriber to Popular Rationalism. For the full experience, become a paying subscriber. And check out our awesome, in-depth, live full semester courses at IPAK-EDU. Hope to see you in class!







Leave a Reply
You must be logged in to post a comment.